This judgment was handed down at 2.00 pm on 28 April 2025 by circulation to the parties' representatives by email and release to The National Archives
- In these proceedings the Claimants each seek declarations that supplementary protection certificates SPC/GB13/021 and SPC/GB14/050 ("the SPCs") are invalid and orders for their revocation, as well as a declaration that European Patent (UK) No. 1506211 ("the Patent"), which was the basic patent on which the SPCs were granted, was invalid. The only ground of invalidity of the SPCs which remains live is that the Patent was invalid.
- The Patent was originally in the name of Bristol-Myers Squibb Co. ("BMS") but was assigned to the Defendant ("AZ") in 2014. The Patent claimed a priority date of 20 May 2002 and expired on 14 May 2023. It claimed a compound known as dapagliflozin and its use in the manufacture of a medicament for, inter alia, treating diabetes.
- Dapagliflozin is an inhibitor of the sodium-dependent glucose co-transporter protein SGLT2. SGLT2 is responsible for the re-uptake of glucose in the proximal tubule of the kidney back into the bloodstream. SGLT2 inhibitors are understood to reduce blood glucose levels by preventing glucose reabsorption into the blood, thereby facilitating excretion into the urine.
- Dapagliflozin was first authorised for marketing in the UK for the treatment of type II diabetes as monotherapy on 14 November 2012 and, in combination with metformin, on 21 January 2014. SPC/GB13/021 relates to dapagliflozin and (with its associated paediatric extension) is due to expire on 13 May 2028. SPC/GB14/050 relates to a combination of dapagliflozin and metformin and is due to expire on 14 May 2028. Dapagliflozin has been very successful commercially; hence the Claimants' interest in invalidating the SPCs.
- The Claimants contend that the Patent was invalid for lack of inventive step and/or insufficiency. In summary they plead that:
(a) the Patent did not make it plausible that dapagliflozin is an SGLT2 inhibitor, a selective SGLT2 inhibitor or useful for treatment of diabetes; and
(b) the Patent did not make a technical contribution over a BMS PCT application published on 19 April 2001, namely WO 01/27128 A1 ("WO 128"), but rather merely made an arbitrary selection of dapagliflozin from the class of compounds disclosed in WO 128 without disclosing any advantage for dapagliflozin compared to that class.
Originally the Claimants contended that it was in any event obvious (in the conventional sense) to arrive at dapagliflozin as an SGLT2 inhibitor from the disclosure of WO 128, but that allegation was not pursued in their closing submissions.
- At trial, Mr Mitcheson KC conducted the oral advocacy for the Claimants and Ms Lane KC did likewise for AZ. Given that all the issues in this case were closely related, it was entirely understandable that the parties decided, after due consideration, not to allocate any part of the oral advocacy to junior counsel. I am grateful to all counsel and to all the firms of solicitors involved for their work in preparing the evidence and submissions.
The Law
- This case raises once again the question of the nature and extent of the disclosure which is required in order for a patent for a chemical compound to satisfy the twin statutory requirements of inventive step and sufficient disclosure. As has often been emphasised, the fundamental principles underlying the requirements of inventive step and sufficient disclosure are that patents are granted for technical contributions to the art (or, to put it another way, for technical solutions to problems) and that the claimed monopoly must be justified by such a technical contribution. The task facing tribunals has been to work out how those principles apply, in the context of the twin statutory requirements, to patents for chemical compounds which are said to have a particular utility.
- I shall address the case law under two heads, reflecting the way in which the parties presented it (and using the headings which they adopted), though the two strands of case law and the principles which they reflect are in fact interwoven.
Plausibility
The Warner-Lambert line of cases
- The case law of the Boards of Appeal of the European Patent Office ("EPO") and of the courts of this jurisdiction has been reviewed in a number of judgments of the Court of Appeal (most recently in the judgment of Arnold LJ in Sandoz v Bristol-Myers Squibb [2023] EWCA Civ 472 ("Apixaban CA")) and by the Supreme Court in Warner-Lambert v Generics [2018] UKSC 56. It is not necessary to traverse all that ground again, particularly as it is common ground that I am bound by the judgments in those cases to apply the standard set out by the majority in Warner-Lambert. It is, however, worth highlighting certain aspects of the authorities.
- The starting point in the relevant line of authorities is generally regarded as being T 939/92 AgrEvo / Triazoles. In that case the application claimed a class of compounds defined by a Markush formula, and asserted that the claimed compounds had herbicidal activity. The Board emphasised (at paragraph 2.4.2) the general legal principle that a patent monopoly should be justified by the technical contribution to the art, and held that this principle applied to the requirement of inventive step (Article 56 EPC) as much as it did to the requirement of sufficient disclosure (Article 83 EPC). It observed (at paragraph 2.5) that if claimed compounds did not have a technically useful property, then the "problem" solved by their provision would be the minimalist one of merely providing further compounds, which was not inventive. That is because "structural originality [has] no intrinsic value or significance for the assessment of inventive step as long as it [does] not manifest itself in a valuable property in the widest sense, an effect or an increase in the potency of an effect" (see paragraph 2.5.1). The Board said (at paragraph 2.5.3) that in light of the general legal principle, in order for a selection of compounds to be patentable, it "must not be arbitrary but must be justified by a hitherto unknown technical effect which is caused by those structural features which distinguish the claimed compounds" from other compounds. It went on to say (at paragraph 2.6) that a technical problem could only be accepted as having been solved if it would be credible that substantially all the compounds claimed possessed the relevant technical effect.
- In T 609/02 Salk Institute / AP-1 complex the claim was in Swiss form (i.e. to the use of a compound for the manufacture of a medicament for a particular therapeutic application). At paragraphs 8-10, the Board explained the policy reasons for requiring an application for such a patent to disclose sufficient information to make it at least plausible that the compound would be effective to treat the relevant disease, and provided guidance as to the nature and extent of the information that would be required for that purpose. The whole passage is worth recalling (citations omitted, emphasis added in paragraph 9):
"8. ...Sufficiency of disclosure must be satisfied at the effective date of the patent, ie on the basis of the information in the patent application together with the common general knowledge then available to the skilled person. Acknowledging sufficiency of disclosure on the basis of relevant technical information produced only after this date would lead to granting a patent for a technical teaching which was achieved, and, thus, for an invention which was made, at a date later than the effective date of the patent. The general principle that the extent of monopoly conferred by a patent should correspond to, and be justified by, the technical contribution to the art, has to be kept in mind...
9. Where a therapeutic application is claimed ... in the form of the use of a substance or composition for the manufacture of a medicament for a defined therapeutic application, attaining the claimed therapeutic effect is a functional technical feature of the claim.... As a consequence, under Article 83 EPC, unless this is already known to the skilled person at the priority date, the application must disclose the suitability of the product to be manufactured for the claimed therapeutic application. It is a well-known fact that proving the suitability of a given compound as an active ingredient in a pharmaceutical composition might require years and very high developmental costs which will only be borne by the industry if it has some form of protective rights. Nonetheless, variously formulated claims to pharmaceutical products have been granted under the EPC, all through the years. The patent system takes account of the intrinsic difficulties for a compound to be officially certified as a drug by not requiring an absolute proof that the compound is approved as a drug before it may be claimed as such. The boards of appeal have accepted that for a sufficient disclosure of a therapeutic application, it is not always necessary that results of applying the claimed composition in clinical trials, or at least to animals are reported. Yet, this does not mean that a simple verbal statement in a patent specification that compound X may be used to treat disease Y is enough to ensure sufficiency of disclosure in relation to a claim to a pharmaceutical. It is required that the patent provides some information in the form of, for example, experimental tests, to the avail that the claimed compound has a direct effect on a metabolic mechanism specifically involved in the disease, this mechanism being either known from the prior art or demonstrated in the patent per se. Showing a pharmaceutical effect in vitro may be sufficient if for the skilled person this observed effect directly and unambiguously reflects such a therapeutic application ... or, as decision T 158/96 also put it, if there is a "clear and accepted established relationship" between the shown physiological activities and the disease... Once this evidence is available from the patent application, then post-published (so-called) expert evidence (if any) may be taken into account, but only to back-up the findings in the patent application in relation to the use of the ingredient as a pharmaceutical, and not to establish sufficiency of disclosure on their own.
10. The appellant argued that experimental tests were in fact irrelevant because no prediction could be made on their basis that the observed effect would equally be seen in vivo. The board will agree that an in vitro effect may not necessarily be reflected in vivo, but this does not lessen the usefulness of in vitro tests in general in relation to sufficiency of disclosure. Indeed, the in vitro tests cannot be performed unless the "protagonists" of the test are available. This means that the skilled person is made aware of the structure of the active ingredient proposed for the pharmaceutical composition as well as, in technical terms, of a definite link between the ingredient and the mechanism allegedly involved in the disease state. The presence of a cause/effect relationship is, thus, made plausible. For how incomplete the data might be, they nonetheless go one step further towards disclosing the invention without leaving an undue burden to the reader. In this context, it should be noted that it is on the very same kind of tests (but published some three to four years later) that the appellant based its arguments in favour of sufficiency of disclosure. In any case, the appellant's argument could not justify the recognition of sufficiency of disclosure in relation to a claim to a therapeutic application of a composition when in the specification there exists no evidence at all of its potential effectiveness."
- The policy reasons referred to in paragraph 8 of Salk were reiterated by the Board in T 1329/04 Johns Hopkins / GDF-9, where the claim was to a polynucleotide encoding a particular polypeptide which was asserted to be a member of the TGF-b superfamily and hence to have activity as a growth differentiation factor. At paragraph 10 the Board explained that in a first to file system it was particularly important that "the application allows to conclude that the invention had been made, i.e. that a problem had indeed been solved, not merely put forward at the filing date of the application." It went on to explain in paragraph 12 that "The definition of an invention as a contribution to the art, i.e. as solving a technical problem and not merely putting forward one, requires that it is at least made plausible by the disclosure in the application that its teaching solves indeed the problem it purports to solve."
- In his judgment in Warner-Lambert Lord Sumption (with whom Lord Reed and Lord Briggs agreed on this aspect) considered these and other significant decisions of the Boards of Appeal as well as the evolution of the case law in this jurisdiction. It is not necessary to set out the whole of that analysis, but I should remind myself of the principles set out by Lord Sumption in [36]-[37] (with emphasis and line breaks added), as it is common ground that these are the principles which I need to apply:
"36. The Court of Appeal's statement of the effect of the plausibility test has already been quoted (para 20 above). They considered that the threshold was not only low, but that the test could be satisfied by a "prediction ... based on the slimmest of evidence" or one based on material which was "manifestly incomplete". Consistently with that approach, they considered (paras 40, 130) that the Board's observations in SALK laid down no general principle. I respectfully disagree. The principle is that the specification must disclose some reason for supposing that the implied assertion of efficacy in the claim is true. Plausibility is not a distinct condition of validity with a life of its own, but a standard against which that must be demonstrated. Its adoption is a mitigation of the principle in favour of patentability. It reflects the practical difficulty of demonstrating therapeutic efficacy to any higher standard at the stage when the patent application must in practice be made. The test is relatively undemanding. But it cannot be deprived of all meaning or reduced, as Floyd LJ's statement does, to little more than a test of good faith. Indeed, if the threshold were as low as he suggests, it would be unlikely to serve even the limited purpose that he assigns to it of barring speculative or armchair claims.
37. Plausibility is not a term of art, and its content is inevitably influenced by the legal context. In the present context, the following points should be made.
First, the proposition that a product is efficacious for the treatment of a given condition must be plausible.
Second, it is not made plausible by a bare assertion to that effect, and the disclosure of a mere possibility that it will work is no better than a bare assertion. As Lord Hoffmann observed in Conor Medsystems Inc v Angiotech Pharmaceuticals Inc [2008] RPC 28, para 28, "it is hard to see how the notion that something is worth trying or might have some effect can be described as an invention in respect of which anyone would be entitled to a monopoly".
But, third, the claimed therapeutic effect may well be rendered plausible by a specification showing that something was worth trying for a reason, ie not just because there was an abstract possibility that it would work but because reasonable scientific grounds were disclosed for expecting that it might well work. The disclosure of those grounds marks the difference between a speculation and a contribution to the art. This is in substance what the Technical Board of Appeal has held in the context of article 56, when addressing the sufficiency of disclosure made in support of claims extending beyond the teaching of the patent. In my opinion, there is no reason to apply a lower standard of plausibility when the sufficiency of disclosure arises in the context of EPC articles 83 and 84 and their analogues in section 14 of the Patents Act. In both contexts, the test has the same purpose.
Fourth, although the disclosure need not definitively prove the assertion that the product works for the designated purpose, there must be something that would cause the skilled person to think that there was a reasonable prospect that the assertion would prove to be true.
Fifth, that reasonable prospect must be based on what the TBA in SALK (para 9) called "a direct effect on a metabolic mechanism specifically involved in the disease, this mechanism being either known from the prior art or demonstrated in the patent per se."
Sixth, in SALK, this point was made in the context of experimental data. But the effect on the disease process need not necessarily be demonstrated by experimental data. It can be demonstrated by a priori reasoning. For example, and it is no more than an example, the specification may point to some property of the product which would lead the skilled person to expect that it might well produce the claimed therapeutic effect; or to some unifying principle that relates the product or the proposed use to something else which would suggest as much to the skilled person.
Seventh, sufficiency is a characteristic of the disclosure, and these matters must appear from the patent. The disclosure may be supplemented or explained by the common general knowledge of the skilled person. But it is not enough that the patentee can prove that the product can reasonably be expected to work in the designated use, if the skilled person would not derive this from the teaching of the patent."
- AZ emphasised that experimental data is not necessarily required to satisfy this test (as was common ground and as is clear from Warner-Lambert). In particular it reminded me of Illumina Cambridge v Latvia MGI. In that case Example 1 stated that nucleotides bearing a 3' O-azidomethyl blocking group "have been shown to be successfully incorporated by a number of different polymerases, block efficiently, and may be subsequently removed under neutral, aqueous conditions using water soluble phosphines or thiols allowing further extension". No graphs or gels were provided but, as Birss J said, that was "a statement that experiments have been done and that they were successful in various specific ways which are relevant to success from the point of view of the skilled person"; he went on to hold that the information provided was plausible and supported the idea that the scheme based on the use of the 3' O-azidomethyl blocking group would work (see [2021] EWHC 57 (Pat) ("Illumina HC") at [238]-[241]). On appeal, Arnold LJ held that Birss J was correct to hold that Example 1 was a statement that experiments had been done and were successful in various specific ways, and that he was entitled to find, on the evidence, that the information provided in Example 1 made it plausible that an 3' O-azidomethyl blocking group would work (see [2021] EWCA Civ 1924 ("Illumina CA") at [106] & [116]).
- Relatedly, AZ reminded me of Evans Medical's Patent [1998] RPC 517 where Laddie J said, at p.550, that if a patent document described an experiment and its results, it did not matter whether the experiments had in fact been carried out and the results obtained. As he said, "what is important is what the document teaches, not how the contents got there". In Sandoz v Bristol-Myers Squibb [2022] EWHC 822 (Pat) ("Apixaban HC") at [138] Meade J agreed that Evans Medical meant that one cannot go behind a statement of fact in a patent document about what was done, but it did permit challenge to the validity of an inference said to be based on it.
- These cases show that (at least in the absence of something which casts real doubt about the veracity of the statement - see Arnold LJ in Illumina CA at [108]) a statement in a patent document that an experiment was done and certain results were obtained is to be taken at face value. Further, it may be, as Illumina shows, that a statement about the experiment and the results is sufficient to make the relevant technical effect plausible, even if the results are presented in verbal form without numerical or graphical data.
- However, what is important is the disclosure of the document. It is important to examine the disclosure to see whether the document does in fact contain a statement that an experiment was done and certain results obtained, or whether it contains no more than a bare assertion that the compound has a particular property. If the document does not contain a statement that an experiment was done and certain results obtained, then (just as it cannot be permissible to go behind such a statement) it cannot be permissible to assume that the patentee had done an experiment and obtained data to support what would otherwise be a bare assertion - see Henry Carr J in Actavis v Lilly [2015] EWHC 3294 (Pat) at [183]-[184].
- Similarly, if the document does not contain a statement that an experiment was done and certain results obtained, it is nihil ad rem that the document discloses an assay which could readily be carried out by the skilled person to determine whether the compound does indeed have the property which is asserted - see Apixaban CA at [95].
- AZ also emphasised that the Warner-Lambert test does not necessarily require any data to be in vivo data. It only requires information (whether in the form of experimental data or a priori reasoning) establishing a reasonable prospect that the assertion would prove to be true based on "a direct effect on a metabolic mechanism specifically involved in the disease, this mechanism being either known from the prior art or demonstrated in the patent per se." That, as is clear from Salk, may be satisfied by in vitro data if there is a "clear and accepted established relationship" between the relevant activity and the disease.
- Relatedly, AZ submitted that Warner-Lambert and Salk are considering efficacy rather than other factors which may affect whether a compound turns out to be suitable for administration to humans, such as side effects and issues of ADME (absorption, distribution, metabolism and excretion). In support of that, it referred me to what Birss LJ said in Akebia v Fibrogen [2021] EWCA Civ 1279 ("Fibrogen CA") at [54], where he explained that the reasonableness of a prediction of utility to treat a disease is unlikely to be falsified by "the fact that active compounds within the formula turn out to be unsuitable as clinically approved agents for reasons unrelated to efficacy itself, such as side effect profiles, bioavailability and the like, depending again on this being a matter of degree".
- A similar focus on efficacy is to be found in the judgment of Meade J in Apixaban HC (see [86]-[93] and [219]-[221]). In that case one argument advanced by the claimants was that the plausibility of apixaban for use in therapy had not been established because it was not plausible that apixaban was selective for factor Xa as compared to other serine proteases. It was argued that a lack of selectivity did not just risk side effects, but went to efficacy, because of the involvement of other serine proteases in the coagulation cascade. Meade J rejected that, because a lack of selectivity would just mean a risk of reduced overall efficacy as a result of off-target effects on other serine proteases and would not mean that overall efficacy was not plausible (if otherwise it had been).
- I accept that the focus should be on plausibility of efficacy rather than side effects, and that selectivity is relevant only if, without a suitable level of selectivity, it is not plausible that a compound will have useful efficacy.
- However, Warner-Lambert and Salk also emphasise the importance, if in vitro data is to be relied on, of the relevant activity demonstrating "a direct effect on a metabolic mechanism specifically involved in the disease, this mechanism being either known from the prior art or demonstrated in the patent per se." In other words, the in vitro data must make it plausible that the compound will treat the disease, even if it may ultimately turn out to be unsuitable, because of the known or demonstrated relationship between the activity and the disease. That emphasises the importance of there being a relationship, whether already known to the skilled person or demonstrated in the patent, such that it can be said that, given the in vitro data, it is plausible that the compound will be efficacious to treat the disease. In any case, it will be necessary to consider the extent to which it is possible, given what is known to the skilled person or demonstrated in the patent, to make a reasonable prediction (at the Warner-Lambert standard) of efficacy in vivo and utility to treat the disease. One factor which may be relevant is the level of knowledge about the correlation between in vitro and in vivo effects (which may depend upon ADME factors).
- In Warner-Lambert Lord Sumption addressed plausibility on the basis of the disclosure of the patent. Arnold LJ has explained why in practice it is appropriate to address plausibility on the basis of the application as filed (or the priority document, if priority is claimed), because if plausibility arises only because of matter which is not present in the application (or the priority document) then there is added matter (or loss of priority) - see Illumina CA at [97]-[100] and Apixaban CA at [53]. In this case, AZ originally relied in support of its case on plausibility on the abstract in the application for the Patent, which was not present in the Patent itself. The Claimants submitted that it was impermissible to rely on the abstract of the application for the reasons given by Arnold J in Abbott v Medinol [2010] EWHC 2865 (Pat) at [65]-[70]. In the light of that, AZ abandoned reliance on the abstract. It would, in any event, be strange if it were permissible to establish plausibility of a claimed invention by reference to material which was present in the application but not present in the patent. In the present case, once the abstract is ignored, there is no difference between the Patent and its application, and so it is possible to consider only the Patent.
- In Apixaban CA, the Court of Appeal had to address the question of whether the approach of the majority in Warner-Lambert was limited to second medical use claims. The Court of Appeal held that it also applied to claims to single compounds per se. Arnold LJ explained why in [85] of his judgment:
"It is true that, as Lord Sumption noted at [23], the concept of plausibility originated as a response to over-broad claims, in particular claims to whole classes of compounds, as in Agrevo. Idenix is an example of its application in that context by the courts of this country. It is also true that, as Lord Sumption noted at [19]-[20], that the concept was also found to be of utility in addressing one of the problems with second medical use claims. Nevertheless the concept was applied by the Board of Appeal to a claim to single compound in BMS/Dasatinib, which was one of the cases relied upon by Lord Sumption (and one of the cases reviewed by the Enlarged Board in G 2/21). As the Claimants point out, the present case is strikingly similar to BMS/Dasatinib. Moreover, BMS/Dasatinib does not stand on its own, because the claim in Johns Hopkins, which was another of the cases relied upon by Lord Sumption and reviewed by the Enlarged Board, was effectively a claim to a specific molecule. The concept has also been applied by this Court in Generics v Yeda to a claim to what was in substance a single product, albeit a product comprising a mixture of polypeptides. Furthermore, the underlying principles are applicable as much to claims to single chemical compounds as to claims to classes of compounds and second medical use claims. The fundamental principle is that the scope of the patent monopoly must be justified by the patentee's technical contribution to the art. This remains so whether the scope of the claim is broad or narrow. Thus when considering inventive step it is necessary to consider what technical problem the claimed invention solves. If it is not plausible that the invention solves any technical problem then the patentee has made no technical contribution and the invention does not involve an inventive step. Equally, when considering insufficiency it is necessary to consider whether the specification sufficiently discloses the claimed invention. If it is not plausible that the invention solves any technical problem then the patentee has made no technical contribution and the specification does not disclose any invention. It follows that, in order for a claim to a single chemical compound to be patentable, the application must make it plausible, when read in the light of the skilled person's common general knowledge, that the compound has the utility asserted for it. Moreover, it makes no difference whether the claim incorporates the use of the compound as a technical feature or whether the claim is simply to the compound per se and the assertion of utility is only to be found in the specification. This is because, as explained above, there is no invention in merely identifying a new chemical compound; invention can only lie in identifying its utility."
- Before one can apply the approach of the majority in Warner-Lambert it is necessary to decide what technical effect is under consideration. The Court of Appeal provided a structured approach to this in Fibrogen CA. At [51] Birss LJ cited from the judgment of Kitchin LJ in Regeneron v Genentech [2013] EWCA Civ 93, including at [100]-[101]:
"100. It must therefore be possible to make a reasonable prediction the invention will work with substantially everything falling within the scope of the claim or, put another way, the assertion that the invention will work across the scope of the claim must be plausible or credible. The products and methods within the claim are then tied together by a unifying characteristic or a common principle. If it is possible to make such a prediction then it cannot be said the claim is insufficient simply because the patentee has not demonstrated the invention works in every case.
101. On the other hand, if it is not possible to make such a prediction or if it is shown the prediction is wrong and the invention does not work with substantially all the products or methods falling within the scope of the claim then the scope of the monopoly will exceed the technical contribution the patentee has made to the art and the claim will be insufficient. It may also be invalid for obviousness, there being no invention in simply providing a class of products or methods which have no technically useful properties or purpose."
- Birss LJ continued at [52]-[53]:
"52. It may be a matter of taste only but I prefer to refer to this fourth principle as reasonable prediction rather than simply plausibility, however whatever it is called, it is the same principle.
53. To apply the reasonable prediction principle one has to take three steps. First one must identify what it is which falls within the scope of the claimed class. Second one must determine what it means to say that the invention works. In other words what is it for? Once you know those two things, the third step can be taken: to answer the question whether it is possible to make a reasonable prediction the invention will work with substantially everything falling within the scope of the claim."
- As Birss LJ went on to explain, the first step is one of claim construction, while the third step involves the application of the approach of the majority in Warner-Lambert. At [57] he made the following observations about the second step in a case where the claim itself does not identify any technical effect:
"In some cases the second step is the aspect which is a bit more involved. So in Idenix v Gilead, claim 1 was to a Markush class of molecules (see Kitchin LJ para [61]). The claim language did not include any reference to what they were for and so one could not answer the question at the second step by looking at the words of the claim. This is also not unusual. If the compounds are new, then a claim to those compounds will be novel without including a claim feature which refers to what they are actually for. However that does not prevent the reasonable prediction principle being applied. In fact the answer in Idenix was clear from the patent specification. That showed that the point of the invention was to treat infections caused by viruses in the Flaviviridae family. So one can assess the validity of the claim on the basis that it is a claim to compounds with anti-Flaviviridae activity, which is what Kitchin LJ said at paragraphs [113] and [124]. So, in the language coined above, anti-Flaviviridae activity was a step two functional feature. The issue in Idenix arose in the context of inventive step but the same approach applies to reasonable prediction/plausibility. Note that this does not mean that claims to compounds per se are actually limited to using the compounds for treating Flaviviridae infections, but for the purposes of assessing questions like inventive step and reasonable prediction/plausibility, one needs to know what the compounds are supposed to be useful for. In fact in Idenix the outcome of the third step was against the patentee. The court held that it was not plausible that substantially all the claimed molecules would be effective against Flaviviridae infections, and hence it was Agrevo obvious and also insufficient for lack of plausibility for the same reason (see paragraphs [129] and [140])."
- AZ suggested that the identification of the technical effect in the case of a compound claim is an aspect of claim construction, relying on Pharmacia v Merck [2001] EWCA Civ 1610. I do not agree that the Court of Appeal in Pharmacia was construing the compound claim as incorporating the technical effect as a functional feature. That can be seen from the way in which they addressed novelty of the compound claim (see Aldous LJ at [104]-[121] and Arden LJ at [193]-[200]) - they held the compound claim to be anticipated by the prior disclosure of a class of chemical compounds without considering whether the prior art disclosed the technical effect. In any event, the point is put beyond doubt by Fibrogen CA, which treats step 2 as distinct from the issue of claim construction in step 1 (and see the pre-penultimate sentence in [57], quoted above).
- In Apixaban HC, Meade J had to address the question of whether a patentee is permitted to rely on a technical contribution which is more limited than the most ambitious assertion in the patent. At [66] & [68] he said this:
"66. In my view BMS is right overall on this point, and in cases where the objection is of lack of plausibility in an Agrevo-type situation, a patentee is not necessarily limited to the most demanding teaching of utility in the specification and is entitled to try to rely on a less ambitious degree of utility, or a utility of a different but related kind.
...
68. So I conclude that what it means for the invention to "work" is to be determined from the specification where the claim is not explicit (I do not think this in itself was in dispute), but that the patentee is not restricted to the most ambitious assertion made. In some cases the patentee may be able to rely on a more limited contribution, but this must be fact-dependent and will still have to find a basis in the specification."
- However, there are limits to how low a patentee can go in pitching its technical contribution. As Meade J said in Apixaban HC at [76], the law requires a technical contribution of some, if low, real significance and there is no contribution in disclosing a uselessly low degree of activity. Similarly, it is not a technical contribution for something to lack practical utility but to be a starting point for research that it is hoped would lead to something which does have utility (see Apixaban HC at [232]). In Gilead v NuCana [2023] EWHC 611 (Pat) Meade J had to consider whether it was enough for a compound to be a "research tool". He held that it was not enough for a compound to be one that could be used to generate information in the form of success or failure in an assay, which information could then be fed into a research project exploring mechanisms of action or structure-activity relationships. However, he distinguished such a compound from one that is itself useful to do something, such as a physical tool that can be used to accomplish a task. See [333], [382] and [389].
G 2/21 and its aftermath
- While accepting that in the light of Apixaban CA I was bound to apply the approach of the majority in Warner-Lambert, AZ reminded me of the decision of the Enlarged Board in G 2/21. In that case the referring board had identified two lines of case law of the Boards of Appeal, applying what it called "ab initio plausibility" (an approach which it aligned with that of the majority in Warner-Lambert) and "ab initio implausibility" (an approach which it aligned with that of the minority in Warner-Lambert). The Enlarged Board eschewed the use of the term "plausibility" and instead answered the questions referred to it using a different form of words which it said were to be applied when considering cases under Article 56 EPC:
"93. The relevant standard for the reliance on a purported technical effect when assessing whether or not the claimed subject-matter involves an inventive step concerns the question of what the skilled person, with the common general knowledge in mind, would understand at the filing date from the application as originally filed as the technical teaching of the claimed invention. The technical effect relied upon, even at a later stage, needs to be encompassed by that technical teaching and to embody the same invention, because such an effect does not change the nature of the claimed invention.
94. Hence, a patent applicant or proprietor may rely upon a technical effect for inventive step if the skilled person, having the common general knowledge in mind, and based on the application as originally filed, would consider said effect as being encompassed by the technical teaching and embodied by the same originally disclosed invention."
The wording in paragraph 94 was repeated in paragraph 2 of the order, with the word "consider" replaced by "derive".
- The Enlarged Board recognised the "abstractness" of the criteria it had advanced (see paragraph 95) and subsequent decisions have arrived at different conclusions as to the meaning of the decision in G 2/21. In Apixaban CA at [94] Arnold LJ expressed the view (obiter, as AZ pointed out) that the Enlarged Board's approach was much closer to "ab initio plausibility" than "ab initio implausibility". In T 118/16 Sumitomo / Insecticide compositions the board which had made the reference in G 2/21 interpreted the Enlarged Board as having adopted a standard which required consideration of whether the skilled person would have "legitimate reason to doubt that the purported technical effect can be achieved with the claimed subject-matter" (see paragraph 11.11). However, in T 314/20 Boehringer Ingelheim / Glucopyranosyl-substituted benzene derivative a different board identified what it regarded as a number of problems with the reasoning in T 118/16 (see paragraph 6.13). It continued at paragraph 6.14:
"In the present case, the current Board does not need to give a definitive answer as to whether it can endorse all the conclusions of decision T 116/18 regarding the two requirements set out in point 2 of the order of decision G 2/21. The Board considers that the purpose of these requirements is to prevent patents from being granted for inventions that are not complete at the filing date. Such speculative applications arise where either the existence of the claimed technical effect or its generalisation is speculative. This may occur because relevant data have not yet been generated or, if available to the patent applicant, have not been disclosed in the patent application."
- In its opening skeleton argument, AZ indicated that it reserved the right to argue in higher courts that Warner-Lambert should be departed from in the light of G 2/21, and asked me to make findings on the evidence both on the Warner-Lambert standard and on the G 2/21 standard. During AZ's oral opening I suggested that, given the difficulties which had been experienced in deciding what the Enlarged Board meant in G 2/21, AZ should instead identify the findings of fact that it wanted me to make (over and above those which would be relevant to the application of the Warner-Lambert standard) so that if a higher court were to hold that the Warner-Lambert standard should be departed from and replaced with a different test in the light of G 2/21, it could apply that test to the facts, regardless of the nature of the test it regarded as appropriate in the light of G 2/21.
- AZ agreed to do that, but the "fact" that it then asked me to find (in a document submitted before the evidence commenced) was whether the technical contribution which it asserted was "encompassed by the technical teaching [of the application] and embodied by the same originally disclosed invention". In other words, it simply asked me to make a finding of "fact" using the wording of paragraph 94 of G 2/21. I asked what AZ said those words meant, and in closing AZ pointed me to extracts from paragraphs 4.1.2 and 4.3.3 of the Case Law of the Boards of Appeal which referred to boards adopting a test of whether a technical effect was "derivable from the original application" and submitted that the test was "pretty basic: can you find the technical effect relied on in the application?" As I understood it, AZ's position was that the test was a purely textual one; in its oral closing, AZ said that whether or not this test was satisfied was a matter of submission based on the application rather than one requiring expert evidence. I cannot see how the test advanced by AZ would achieve the objective identified by the boards in both T 116/18 and T 314/20 of preventing speculative applications. It would allow an applicant to make a bare assertion of a technical effect, even one which would be ab initio implausible, and that would be enough to allow it to rely on the technical effect to support inventive step. However, I shall say no more about this, given that it is not for me to form a conclusion about the nature of the approach in G 2/21, and AZ made it clear in closing that I did not need to make any additional findings of fact in order to allow it to argue in a higher court that the test which it advocated was satisfied.
- In its oral opening AZ said that it was not arguing that G 2/21 had adopted an "ab initio implausibility" standard. However, in the document it submitted prior to the start of the evidence, it asked me to make a finding as to whether the skilled person "would have had legitimate reason to doubt" that AZ's asserted technical contribution could be achieved with the claimed subject-matter. While the Claimants objected to this, I do not think they can really have been surprised by AZ's proposal (leaving aside the change in position from AZ's oral opening the previous day) - after all, Meade J took a similar course in Gilead v NuCana (see [342]) and the approach was trailed in AZ's evidence. However, again AZ did not identify any findings of primary fact which it said I should make (over and above those relevant to application of the Warner-Lambert standard) to enable a "legitimate reason to doubt" test to be applied.
- G 2/21 is concerned only with inventive step. However, in paragraph 77, in a passage which in UK terms would be regarded as obiter, the Enlarged Board said this:
"The reasoned findings of the boards of appeal in the decisions referred to above make clear that the scope of reliance on post published evidence is much narrower under sufficiency of disclosure (Article 83 EPC) compared to the situation under inventive step (Article 56 EPC). In order to meet the requirement that the disclosure of the invention be sufficiently clear and complete for it to be carried out by the person skilled in the art, the proof of a claimed therapeutic effect has to be provided in the application as filed, in particular if, in the absence of experimental data in the application as filed, it would not be credible to the skilled person that the therapeutic effect is achieved. A lack in this respect cannot be remedied by post-published evidence."
- One issue which a higher court considering the impact of G 2/21 on the Warner-Lambert line of case law will have to resolve is whether the standard applicable when considering inventive step should be different from that applicable when considering insufficiency and, if so, why that should be the case and whether the consequence is that claims to products per se (where validity is to be assessed by reference to a disclosed technical effect) are to be treated differently from claims which contain a functional technical feature; in the present case, as will be seen below, the Patent contains claims of both types. But that is not a matter for me - my task is to apply the Warner-Lambert standard and make any findings of fact that I consider may be needed to allow a higher court to apply the "legitimate reason to doubt" standard if it should find that to be the correct test.
Arbitrary selection
- As mentioned in paragraph 10 above, one aspect of the decision in AgrEvo was the Board's statement that for a selection of compounds to be patentable, it "must not be arbitrary but must be justified by a hitherto unknown technical effect which is caused by those structural features which distinguish the claimed compounds" from other compounds.
- In T 133/01 Wyeth / Dopamine agonists the claim was to a particular compound which was said to have dopamine D2 receptor agonist activity. The prior art document disclosed a class of compounds which covered the claimed compound (but did not individually disclose it) and which were also said to have dopamine D2 receptor agonist activity. The applicant argued that the problem to be solved was the provision of a dopamine D2 receptor agonist with better selectivity. However, the experiment submitted by the applicant failed to compare selectivity of the claimed compound with that of any of the compounds which had been individually disclosed in the prior art document. Therefore the technical problem had to be reformulated as providing further compounds having dopamine D2 receptor agonist activity. The Board held (at paragraph 4.6) that the claimed invention lacked inventive step. It observed that the prior art document taught that all the compounds which it covered had dopamine D2 receptor agonist activity, and continued:
"The presumption prevails, therefore, that the selected [compound] of claim 1 will exhibit the same pharmacological activity as that compound represents an arbitrary selection out of a known class of active compounds. In the absence of evidence to the contrary, the Board concludes that faced with the problem indicated above, namely to provide merely further compounds having a dopamine D2 receptor agonist activity, a skilled person would not require any inventive skill in picking out at random from structural variants outlined in document (1) the [relevant substitutions] thereby arriving without inventive ingenuity at the compound of claim 1, which is the solution proposed by the present application."
- In Dr Reddy's v Eli Lilly [2009] EWCA Civ 1362 the patent claimed olanzapine and disclosed its beneficial properties as an anti-psychotic by reference to the results of animal and early clinical studies. The prior art document ("235") contained a Markush formula covering 1019 compounds and a "preferred" class of 86,000 compounds. It went on to assert, without any evidence, that the compounds had neuroleptic, sedative or relaxant effects which made them useful in the treatment of certain forms of anxiety and psychosis.
- The judgments of the Court of Appeal each started by rejecting an attempt to apply, under the Patents Act 1977, the approach to so-called "selection inventions" that had prevailed under the 1949 Act. Having done so, Jacob LJ said at [40] & [44]:
"40. So I think the better approach is to see what the EPO Boards do when a patented product or class of products falls within a greater class. They deploy the objection of obviousness where the patentee has in truth made no real technical advance.
...
44. What then does the EPO do? The answer is essentially this: that it regards what can fairly be regarded as a mere arbitrary selection from a class as obvious. If there is no more than an arbitrary selection then there is simply no technical contribution provided by the patentee."
- He then referred to AgrEvo and Wyeth and continued at [50]-[52]:
"50. ... The EPO jurisprudence is founded firmly around a fundamental question: has the patentee made a novel non-obvious technical advance and provided sufficient justification for it to be credible? That is the basis of all the reasoning – see e.g. [2.4.2] of AgrEvo. A "selection" (by which I mean the later claimed compound or sub-class) which makes a real technical advance in the art is patentable.
51. More specifically Mr Carr contended that a sub-class or individual member of a prior art published class was taken to be obvious if it was a random selection from the earlier class. I have no difficulty with that. Such a "selection" provides no technical contribution. Mankind can learn nothing new from it. Nor indeed does Lilly dispute that proposition. It said in its skeleton argument: "Lilly does not dispute that in relation to obviousness a selection from the prior art cannot be merely arbitrary."
52. Of course one has to consider here what is meant by an "arbitrary selection." The answer is to be found in the guiding principle - is there a real technical advance?"
- Jacob LJ then went on to consider whether "by identifying olanzapine and disclosing what it does about its properties, Lilly were making an arbitrary selection". He answered that question in the negative for two reasons. First, the patent addressed problems that were nothing to do with selecting from the class in 235 and disclosed properties of olanzapine that were superior to those of the closely related compound ethyl olanzapine (which was also covered by 235). Jacob LJ said (at [55]) that: "None of that indicates a mere "arbitrary selection" telling the reader in effect no more than he would get from reading 235." Secondly, 235 merely made vague promises about the properties of all the 1019 compounds, and the skilled reader would know about the unpredictability in the field. Hence, Jacob LJ said at [57]: "Once it is accepted that the whole field is unpredictable, then the woolly teaching of 235, if not totally useless, is no guide to any particular compound. You cannot say a particular compound out of a vast class is obvious if you have no real idea as how any individualised member of that class might behave."
- Lord Neuberger MR, having referred to the decisions of the Boards in AgrEvo, Wyeth and T 181/82 Ciba Geigy / Spiro compounds, said at [109]-[111]:
"109. ...This seems to me to establish that the correct question to ask is whether the selection of olanzapine, out of the class of 86,000 compounds in 235, was "arbitrary", or whether the teaching of the patent established that the selected compound achieved "a particular technical result", and, in answering that question, one must bear in mind that it arises in the context of the broader proposition that "the extent of a patent monopoly should correspond to and be justified by the technical contribution to the art".
110. Whether one looks at the broader proposition or the narrower question, it appears to me that the answer is, unsurprisingly, the same. There can be no doubt but that the teaching of the patent in relation to a single compound, as described by Jacob L.J. in paras. 7 to 10, is unusual in its extent, as he points out in paras. 11 to 13, and it was at least open to the judge to conclude that it represented a significant technical contribution to the art over and above the "teaching" (which is a generous description of what appears to be no more than mere speculation about a wide collection of different possible applications of an enormous number of compounds) of the 235 patent.
111. As to the narrower question, I do not consider that it can be said that the selection of olanzapine was arbitrary. There is no doubt that the patent credibly reveals that that single selected compound has technical applications or features which represent a contribution to the art, wholly absent from 235's generalised and unsupported claims for 86,000 compounds which include the selected compound, although it is not referred to specifically. The patent's disclosure is not merely enormously more specific, in terms of both identifying the right compound and its technical application, than 235, but, unlike 235, but it also reports experimental evidence to support the claim. It is true that there is only limited evidence to show that no other compound claimed by the 235 patent has the same therapeutic benefits as olanzapine, but I do not consider that that can invalidate the patent. ..."
- He then went on to refer to the EPO practice, as illustrated by T 181/82 (also cited in Wyeth), of treating a comparison between the claimed compound and the structurally most similar compound in the prior art as satisfying the requirement of showing that the claimed compound had an advantage over the prior art. He accepted that the results in the patent showing an improvement compared to ethyl olanzapine should be taken as satisfying any such requirement. See [112]-[114].
- In Generics v Yeda [2013] EWCA Civ 925, Floyd LJ, having considered AgrEvo and Dr Reddy's, amongst other authorities, included the following in his summary of principles at [50]: "A selection from the prior art which is purely arbitrary and cannot be justified by some useful technical property is likely to be held to be obvious because it does not make a real technical advance."
- In Takeda v Roche [2019] EWHC 1911 (Pat) Birss J said at [203]:
"The law is clear enough that a ground of invalidity exists which can be called different things including: lack of technical contribution, Agrevo obviousness, and failure to solve the technical problem. Depending on the facts one of these descriptions may be more apt in a given case than another but they are all getting at the same thing. [He then cited T 409/91 Exxon / Fuel Oils and AgrEvo.] The general principle there identified is that the extent of the patent monopoly, as defined by the claims should correspond to the technical contribution to the art. This theme - that the patent monopoly should be justified by the actual technical contribution to the art - has often been referred to with approval in the UK, most recently in the two recent Supreme Court decisions [i.e. Warner-Lambert and Actavis v ICOS [2019] UKSC 15]."
- He went on to explain at [204] that:
"One way in which this principle has been applied in the context of inventive step is to deny validity to a selection from the prior art "which is purely arbitrary and cannot be justified by some useful technical property". Such a selection "is likely to be held to be obvious because it does not make a real technical advance" [citing Generics v Yeda and Dr Reddy's]."
- At [205] Birss J explained that sometimes the argument is put on the basis that the claim makes no technical contribution over an item of prior art and said that this was a legitimate way of putting the argument. Finally, at [207] he explained that in such a case, for each alleged technical contribution over a prior disclosure:
"there are five questions to answer: Is it disclosed in the patent? Is it plausible? Is it true? Is it a technical advance? Does it support claims of the breadth they are?"
- AZ submitted that where a selection was made from a genus disclosed in the prior art it was not necessary for the compound(s) selected to have properties which differed from those of the genus. It relied on Generics v Lundbeck [2009] UKHL 12 and in particular what Lord Neuberger said at [83]:
"...it can be said that the Respondent's technical contribution in this case was to make available, for the first time, a product which had previously been unavailable, namely the isolated (+)-enantiomer of citalopram. On that basis, it would appear to follow that the respondent was entitled to claim the enantiomer."
- However, the circumstances in that case were very different. It was known that racemic citalopram would contain two enantiomers and that it was likely that one would have greater activity than the other. The patentee solved the problem of how to synthesise the enantiomers individually, and showed that the (+) enantiomer was the more active of the two. In those circumstances, one can see why the patentee had made a technical contribution which justified a claim to the (+) enantiomer.
- AZ also relied on what Mellor J said in Accord v University of California [2024] EWHC 2524 [Pat] at [433]:
"Finally, there is no requirement for an invention to have to be "better" than the prior art (and not a sensible way this could be evaluated). A new and non-obvious alternative solution to solve a problem remains patentable. This is made clear in a number of cases at the EPO, see for example the Case Law of the Boards of Appeal and decision T 588/93 where it makes clear that it is not necessary to show substantial or gradual improvement over the prior art, an invention can instead be an alternative solution to a known problem (see also T 179/108 [sic, T 1791/08] at §12.5 and the Case Law book at §4.5)."
- However, this was not directed to a case of a selection from a previously described genus. In the case being addressed by Mellor J, the claimed compound (known as RD162') was related to but different from any previously disclosed compounds (the closest being one known as RD162). The EPO cases referred to by Mellor J are ones in which the invention lay in solving a known problem by adopting a different approach from that recommended in the prior art (and indeed one which the prior art taught away from).
- §4.5 of the Case Law book (to which Mellor J referred) also contains the following passage:
"On the other hand, in T 1179/16 the board noted that if the only contribution of the invention was to propose something different from the prior art (i.e. the provision of an alternative), then it was usually appropriate to consider that the skilled reader would take into account any alternative known in the underlying technical field (unless the closest prior art teaches away from it). The board stated that in such cases it might not be required to justify the selection of a particular solution, because it was assumed that an invention based on incorporating known features for the sole purpose of establishing novelty must be rendered obvious by a corresponding step of selecting any alternative known in the art."
- As I understand it, the Board in T 1179/16 was making the point that if what is claimed is merely said to be an alternative to the prior art rather than something with improved properties, it is not necessary to show a pointer to the particular claimed compound: "all which needs to be justified is that the combination of disclosures [in the prior art] would represent a technically reasonable consideration for the skilled reader, with no further need to justify why the specific combination would be selected (e.g. rather than other alternatives)" (see paragraph 3.4.4). That is consistent with the Board's approach in Wyeth.
- It is also consistent with the presence in the EPO's Guidelines for Examination of the following example of a case in which inventive step is lacking (see §3.1(iv) of the Annex to Chapter VII of Part G):
"The invention consists merely in selecting particular chemical compounds or compositions (including alloys) from a broad field.
Example: The prior art includes the disclosure of a chemical compound characterised by a specified structure including a substituent group designated "R". This substituent "R" is defined so as to embrace entire ranges of broadly-defined radical groups such as all alkyl or aryl radicals either unsubstituted or substituted by halogen and/or hydroxy, although for practical reasons only a very small number of specific examples are given. The invention consists in the selection of a particular radical or particular group of radicals from among those referred to as the substituent "R" (the selected radical or group of radicals not being specifically disclosed in the prior art document since the question would then be one of lack of novelty rather than obviousness). The resulting compounds:
(a) are neither described as having nor shown to possess any advantageous properties not possessed by the prior art examples; or
(b) are described as possessing advantageous properties compared with the compounds specifically referred to in the prior art, but these properties are ones which the skilled person would expect such compounds to possess, so that they are likely to be led to make this selection."
- AZ pointed out that this was only part of the Guidelines, rather than a decision of the TBA, and that the Guidelines made it clear that the examples were for illustrative purposes only and that one should not strive to make cases fit into an example which was not clearly applicable. However, the fact that such an example is to be found in the Guidelines indicates that the EPO regards it as a useful illustration of the application of the principles of its case law, albeit one to be used as a servant not a master.
- Overall, I do not perceive any material difference between the case law of the EPO and the UK case law (which is to be expected, given that the intention of the Court of Appeal in Dr Reddy's was to adopt the EPO approach). The requirement is for the claimed invention to in fact constitute a technical advance and for the patent to disclose enough to make that technical advance plausible. If the patent claims a compound selected from a previously disclosed genus of compounds which are said to have a particular property, then that requirement is not satisfied if the compound does not in fact have some different or improved property compared to those previously individually disclosed (a new effect or an increase in an effect), or the patent does not make such improved property plausible.
The Experts
- The Claimants adduced evidence from Professor Bernard Thorens on biological/pharmacological aspects and from Dr Paul Edwards on medicinal chemistry.
- Prof. Thorens is Professor Emeritus of the University of Lausanne and, until the end of 2024, was the Head of Research for Translational Metabolic Diseases at the Swiss Institute for Bioinformatics, Lausanne. He obtained his undergraduate degree in biochemistry in 1978 and his PhD in 1984 from the University of Geneva. He then worked at MIT (where he began to work on glucose transporters) before moving to the University of Lausanne in 1991, where his work has focused on the study of diabetes mellitus and mechanisms of glucose transport in the organs involved in glycaemia.
- Dr Edwards received a BSc in chemistry in 1989 and a PhD in organic chemistry in 1993 from the University of Leicester. After postdoctoral positions at the University of Minnesota and Cambridge, he began work in industry in 1995. From 1997 to 2003, he worked for Pfizer Global Research & Development in the UK in the Medicinal Chemistry and Lead Discovery Technologies Departments. From 2003 to 2005, Dr Edwards was Director of Medicinal Chemistry for Santhera Pharmaceuticals in Heidelberg, leading the dipeptidyl peptidase IV programme. He subsequently held senior roles at Galapagos NV in Belgium and Boehringer Ingelheim in Canada. Since 2012, Dr Edwards has founded several start-up companies.
- AZ adduced evidence on biological/pharmacological matters from Professor Clifford Bailey and on medicinal chemistry from Professor Barry Potter.
- Prof. Bailey is Emeritus Professor of Clinical Science and Anniversary Professor at Aston University in Birmingham, as well as a Visiting Professor at Ulster University. He obtained a BSc in physiology and biochemistry from the University of Sheffield in 1970, followed by a PhD in the field of endocrine control of glucose metabolism from Aston University in 1973. His research has been wide-ranging but has included work on glucose homeostasis and its role in the pathogenesis of diabetes mellitus. Prof. Bailey worked with BMS as a member of the advisory board involved in the design and conduct of the Phase III clinical development programme for dapagliflozin.
- Prof. Potter is Emeritus Professor of Medicinal and Biological Chemistry in the Department of Pharmacology at the University of Oxford and also a Visiting Professor of Medicinal Chemistry in the Department of Life Sciences at the University of Bath. He obtained a BA in chemistry and a DPhil in 1981 from the University of Oxford. Following postgraduate work in Oxford and Göttingen, he became a Lecturer in Biological Chemistry at Leicester University in 1984. Prof Potter moved to the University of Bath in 1990 as a Lister Institute Research Professor of Medicinal and Biological Chemistry in the School of Pharmacy. In 1998 he co-founded the pharmaceutical start-up company Sterix, which was sold to Ipsen in 2004. Prof. Potter returned to the University of Oxford in 2015 to found the Medicinal Chemistry & Drug Discovery sub-section within the Department of Pharmacology.
- The parties were, to varying extents, critical of the other's experts. AZ pointed out that Prof. Thorens had spent his career working on basic science and had not been involved in drug development. I have borne that in mind, but it does not appear to be of any real significance, given the issues in this case and the role of the expert evidence in assessing those issues.
- AZ accused Dr Edwards of being an advocate for the Claimants' case and said that I should exercise considerable hesitation before placing any reliance on his evidence. I completely reject the attack on Dr Edwards. Indeed I was surprised that it was made, as the foundation for it was extremely flimsy. First, reliance was placed on the fact that Dr Edwards had given evidence for generic companies against BMS in the apixaban litigation in various jurisdictions. That is correct, but it was not suggested to Dr Edwards that he was biased as a result, or that it had affected his approach in this case in any way. Secondly, it was said that he had a tendency to give long answers which sounded like rehearsed speeches and were not responsive to the questions. I do not agree - Dr Edwards was responding to the questions and on occasions felt he needed to explain matters at some length but that did not stray into advocacy for the Claimants' case. Thirdly, reliance was placed on the fact that at one point in his oral evidence he suggested that the structure of dapagliflozin contained a potential toxicophore. He explained that he was responding to a statement made in Prof. Potter's second report. It would have been better if the point had been made in writing if it was to be made at all (and Mr Mitcheson did not pursue the point with Prof. Potter), but I do not think that this incident indicates that Dr Edwards was not discharging his duty to the court. Finally, AZ criticised Dr Edwards for being prepared to make an assumption that he was asked to make by the Claimants' solicitors about assay results obtained during his proposed programme of synthesis based on WO 128. I cannot see how this could be a possible basis for criticism.
- The Claimants pointed out that while Prof. Bailey had some involvement with drug development, it tended to be at the clinical end of development rather than working with medicinal chemists. Again I have borne that in mind but, as with Prof. Thorens, it does not appear to be of real significance in this case. More significantly, the Claimants submitted that Prof. Bailey's involvement with BMS, AZ and dapagliflozin had led to unconscious bias on his part. In particular, the Claimants compared Prof. Bailey's discussion of WO 128 in his reports with his discussion of the Patent. In a number of instances, Prof. Bailey was more positive about passages in the Patent than he was about identical passages in WO 128, even where those passages were part of the background to the respective inventions. Prof. Bailey was extremely frank in his oral evidence - he said that his involvement with BMS, AZ and dapagliflozin meant that he would probably have felt a bit uncomfortable giving evidence adverse to the Patent, and that while he had not intended to be more positive in his reports about passages in the Patent than corresponding passages in WO 128, the fact that he had done so indicated that he could be suffering from unconscious bias. In my judgment it is likely that unconscious bias did play a part in the way in which Prof. Bailey's reports were written, but ultimately that did not matter, given the entirely frank and straightforward way in which Prof. Bailey gave his oral evidence.
- The Claimants accepted that generally Prof. Potter gave his evidence fairly and was trying to assist the court. However, they submitted that on occasions he was at pains to stick to a party line, particularly when asked to consider whether there were doubts about certain matters. I do not agree - in my judgment Prof. Potter gave fair and entirely understandable answers in response to such questions.
- In my view, all the experts were trying to assist the court to the best of their ability, and I thank them for their effort to do so. In the event, the areas of dispute between them, on matters which were for expert evidence rather than purely for the court, were fairly limited.
The (Almost) Agreed CGK
- The parties were agreed that the skilled team would include a medicinal chemist and a person whom the Claimants called a biologist and AZ called a pharmacologist. However, the dispute between the parties was not about whether that person was a biologist or a pharmacologist, but about whether they had a focus on glucose transporter proteins or not. I address that issue, and the dispute about the common general knowledge ("CGK") which turns on it, after I have set out the disclosures of the Patent and WO 128 below.
- However, the parties agreed a statement of the CGK of the skilled team which applied regardless of the outcome of the dispute about the biologist/pharmacologist. It is helpful to have that CGK in mind when considering the Patent and WO 128. I have had regard to the whole of the agreed statement, but certain aspects turned out to be of only peripheral (if any) relevance to the issues and are not necessary to understand my judgment. What follows is an edited version of the agreed statement of CGK, to which I have added by addressing the minor disputes about the CGK which did not depend on the makeup of the skilled team.
Medicinal chemistry
- The skilled medicinal chemist would be familiar with the fundamental principles of organic and medicinal chemistry. The skillset of a medicinal chemist would include knowledge of and experience using chemical reaction mechanisms needed to synthesise new compounds, techniques to purify and characterise compounds, and general principles important to a drug's design and pharmacokinetics (e.g. solubility, absorption, metabolism).
- The skilled medicinal chemist would further be familiar with aspects of sugar chemistry such as nomenclature, numbering of atoms, and stereochemistry. In particular they would be aware that anomerism is a type of stereoisomerism used to refer to the position of substituents at the anomeric (position 1) carbon and that a free monosaccharide can exist as two stereoisomers (or anomers), which are referred to as the α and β anomers and are shown on the left and right respectively below.
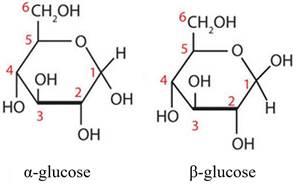
- The bond formed between the anomeric carbon of a sugar and another molecule or fragment is referred to as a glycosidic bond. Once a glycosidic bond is formed the α and β forms cannot freely interconvert under normal conditions.
- Drug discovery and development in general has a series of stages, however in practice drug discovery is an empirical, iterative process where the steps are not rigidly separated and projects do not proceed on such a linear basis, and further the exact process for each stage depends on the specific project and may differ depending on the specific company or laboratory. The stages are:
i) target identification, validation and assay development;
ii) high throughput screening, if appropriate;
iii) generation of novel compounds and "hits";
iv) hit identification, validation and subsequent hit-to-lead;
v) optimisation of lead series or compounds;
vi) further rounds of (iii) to (v), including parallel series of lead series discovery;
vii) pre-clinical development; and
viii) clinical trials.
- In the early stages of (iii) and (iv), the aim is to create as many "hits" or "leads" as possible both in terms of the absolute number of compounds and the number of different sorts (or series) of compounds in the hope that, by investigating and optimising compounds within the lead series, a single compound meeting all the project criteria can be found.
- The overall success rate for new drugs is very low, primarily due to the difficulties of developing new compounds through the drug discovery process. The skilled medicinal chemist may need to design and synthesise hundreds, and sometimes thousands, of compounds to optimise analogues of an initial hit for the interaction of the compounds with the target and other parameters (i.e. multi-parameter optimisation - see below).
Target identification
- The initiation of a drug discovery project frequently starts with the assessment of targets that may have relevance to a specific disease and establishing assays to assess the activities of candidate chemical compounds against this relevant target. The drug target could be an enzyme, receptor, transporter or nucleic acid. Depending on how the drug target is known to cause disease, the drug itself could be an inhibitor/antagonist (which blocks or inhibits the action of the target) or an agonist (which activates the target to produce a response).
- Once identified, a prospective biological target believed to possess a sufficient level of disease linkage is then "validated" in appropriate biological assays. Target validation is achieved by considering biochemical and cell-based models of the disease, as well as identifying suitable animal models, to confirm the relationship between the target and disease.
- The skilled medicinal chemist would also consider whether there was a known 3D structure for the biological target and, if not, whether there was a realistic prospect to obtain one.
- Where a target-disease linkage is known at the outset, a Research Target Profile ("RTP") and/or Target Product Profile ("TPP") may be established which would set out the drug qualities of interest, for example, binding potency to the target, selectivity to the target, oral bioavailability and chemical stability.
Analysing data from developed assays
- At an early stage of the project, the skilled team needs to establish how activity will be measured and which in vitro and in vivo tests will be used. If necessary, assays will be developed for the project. Establishing the appropriate experimental parameters and validation of these tests would also be required.
- A number of assays may be designed and performed to understand various properties of a compound as they proceed through the drug discovery project up until nomination of a clinical candidate, including:
i) potency, often expressed as IC50 (half the maximal inhibitory concentration) or EC50 (half the maximal effective concentration). Lower values indicate a more potent compound;
ii) selectivity, which can be thought of as the ratio of potency of the compound against the desired target versus the potency of the same compound against a different target. For a drug's development, where there are known molecules similar to the target, for which drug activity may or may not be desirable, selectivity would be a relevant factor to consider;
iii) therapeutic activity, being the output of in vivo tests to demonstrate that the compound(s) have activity in an animal disease model;
iv) absorption, distribution, metabolism and excretion ("ADME") assays; and
v) safety data, including toxicity data in e.g. bacterial assays or different cell lines.
Hit identification
- There are a number of ways to identify a suitable chemical starting point. These could include:
i) conducting a literature review to identify known compounds;
ii) conducting a high throughput screen ("HTS");
iii) screening an existing small compound library or starting with a natural product lead or natural product library in an in vitro assay; or
iv) starting from existing/known drugs (where relevant).
- After hits have been identified, they would then be validated using assays developed for the project and criteria set by the skilled team would be used to narrow down the prospective pool of hits. Relevant potential hits would be re-synthesised and tested to remove false positives.
Hit to lead
- This stage involves developing a number of compounds from the initial hit(s) to make analogues (i.e. expanding the hit) to further examine the properties of the hit compound(s) and build up a putative structure-activity relationship ("SAR") to establish which aspects of the molecule could be further developed. Selected compounds developed in this stage will be progressed to the status of a "lead series" or "lead" compounds.
- SAR describes the relationship between the chemical structure of a molecule and its biological activity. It aims to determine which parts of the molecule or functional groups are important for activity, which are not and why. It is used to inform subsequent structural modification efforts. SAR studies involve making small, incremental changes to the chemical structures of the hit compound(s). It was well known that small changes in chemical structure can have a big effect on pharmacological activity and that it was necessary to test a compound to see the effect of a change in structure.
- Once made, analogues would be evaluated to determine the effects of each incremental change on the properties of the compound in order to establish which functional groups are responsible for pharmacological activity and/or other desired properties. The aim at this stage is to build up the 'library' of compounds in order to better understand which groups drive potency, what aspects of those groups appear to be influencing their effect (whether that be electronegativity, electrostatic polarity, size, etc.) and which particular groups of molecular scaffolds may be liabilities.
- SAR analysis is important as it may provide the skilled medicinal chemist with information on which chemical groups within the molecule impact the biological activity of the target. SAR also usually includes analysis of structural property relationships ("SPR") (i.e. how the structure of the molecule impacts its physicochemical and biological properties). Compounds may be screened for (i) activity at the desired target, (ii) selectivity, (iii) toxicity, (iv) metabolism, and (v) solubility, with compounds which do not reach the agreed minimum value in one assay not being progressed (so-called "multi-parameter optimisation").
- SAR studies are an iterative and non-linear process, and may involve designing new compounds for testing based on the information gleaned from the previous generation of compounds. The SAR information is used to make changes to hit compounds to produce several lead molecules or a lead series to be further optimised in the next stage. The process may be envisaged as a cycle.
- The skilled medicinal chemist would have in mind general medicinal chemistry principles in the development of compounds, including Lipinski's "rules", which provided guidance on chemical, physical and calculated properties to be orally bioavailable and "drug-like".
- Chemical groups known to be oncogenic, reactive, unstable or metabolically labile would generally be avoided, as well as potential toxicophores, chemical functional groups that can potentially cause toxicity.
- The hit identification and lead optimisation processes are progressed by investigation of each molecule's structure in the selected series and activity in relevant assays to determine the SAR. Its utility will be determined by the amount of SAR generated around a series of compounds, and the picture of which modifications may give the most desired or undesired effects builds over time as more studies are conducted, and more data are collected.
- If a 3D structure of the target is available (often obtained by X-ray crystallography), this can be used to identify important binding interactions and help to select structural modifications to try. Molecular modelling using homology models may also be used. However, no 3D structure was available for SGLT2.
Lead optimisation
- Once one or more lead compounds/series have been identified, work is then undertaken to improve the activity and other drug-like properties of those compounds and further refining analogues. It is an ongoing iterative process of designing, making and testing the compounds for both activity and other properties such as solubility, metabolism, toxicity, stability, selectivity etc. which operate as a feedback loop.
- During the lead optimisation phase, the SAR work started in the hit-to-lead stage is continued to allow the skilled medicinal chemist to build up a picture of the pharmacophore (i.e. the set of structural and electronic features required for a compound to bind to a biological target). Similar strategies are used, save that the focus is on improving the properties of a specific series of compounds by making smaller changes to the lead compounds / compounds within the lead series' structure.
- Modifications at this stage might include: (i) variation of substituents including functional groups; (ii) extensions to or deletions from the structure; (iii) chain length alterations and potential rigidification strategies; (iv) variations of rings and ring fusions; (v) ring size variations; (vi) use of (bio)isosteres; (vii) generation of prodrugs; and (viii) variations in functionality targeted at improving properties such as lipophilicity and polarity.
- Even at the lead optimisation stage, it is possible that a lead compound or lead series will fail such that no compound or series is found that displays the desired balance of properties. It is therefore usual to progress more than one lead compound/series into optimisation.
Developing the lead
- In this phase, features of the lead compound or lead series which would be investigated/developed would include detailed PK/PD (pharmacokinetics and pharmacodynamics), toxicology, formulation, specificity and safety. For specificity, once the drug discovery project entered lead optimisation, the skilled team would assess for selectivity against other target classes to determine whether there were any undesirable off target effects.
- It was usual for teams involved in drug development to develop one or more back-up candidates or a second lead compound series.
Potency requirements
- As I understood it, there was broad agreement between Dr Edwards and Prof. Potter on this topic. It was common, at the stage of hit identification, to use an in vitro assay with a cut-off of 10 mM (i.e. to detect compounds with an IC50 of 10 mM or less). However, compounds with such a level of potency would not be regarded as acceptable as a drug - for that, medicinal chemists would generally be looking for IC50s in the low nanomolar range or better. One reason for that was to allow headroom for potential ADME issues that may arise in vivo. The aim would be to optimise initial hit compounds, which might have low micromolar potencies, through the hit to lead and lead optimisation processes described above. However, ultimately the required potency would depend on input from the skilled biologist/pharmacologist. I return below to the question of potency requirements in the case of SGLT2 inhibitors.
ADME testing
- There was a minor dispute about the stage in the drug development process at which testing for ADME factors would take place. Dr Edwards and Prof. Potter were agreed that in the mid 1990s the pharmaceutical industry became aware that the failure to address ADME issues at an early stage of development was causing candidate drugs to fail in late stages of development. That led to big pharmaceutical companies studying ADME issues at an earlier stage of development than they had done previously. However, it was also clear that organisations with fewer resources might continue to conduct tests for ADME issues at a later stage. In the end, nothing turned on the precise stage at which a skilled team would conduct ADME testing.
The approach to a diabetes project
- The skilled medicinal chemist's approach to a new diabetes project would be to work with the skilled biologist/pharmacologist to understand the target, and develop an approach to identify a hit and be guided by the wider team. They would then use the data from biological assays to identify hit and lead compounds, and conduct SAR studies to identify further leads and to build up knowledge to assist with the development of the lead(s) as the process progresses.
Biology / Pharmacology
- Glucose is a type of simple sugar (or monosaccharide) that supplies energy to all the cells in the body. It is one of the body's main sources of energy and the primary fuel for the brain, so it is important that a steady source is available. Glucose is transported via the blood and metabolized by cells during respiration to produce adenosine triphosphate (ATP). The amount of glucose in the blood is commonly referred to as the blood glucose level.
- In healthy individuals, blood glucose levels are regulated within a narrow range to maintain a balance between the glucose available in the bloodstream and its removal. Glucose homeostasis, or glycaemic control, is the term used to describe the mechanisms by which glucose levels in the blood are maintained by the body. This process of glycaemic control/glucose homeostasis is necessary in order to ensure adequate glucose is transported to cells throughout the body, while minimizing excess glucose which may be surplus to requirements.
- When there is an excess of glucose (hyperglycaemia), the body stores it in the liver and muscles as glycogen. Conversely, when glucose is in short supply (hypoglycaemia), the body releases glucose from stored glycogen or produces it from other sources. Hyperglycaemia can cause long term damage to tissues and organs, particularly in the cardiovascular and nervous systems. Hypoglycaemia causes fainting, coma or possibly death. The state in which blood glucose is maintained within a stable, normal range is known as euglycaemia.
- The organs involved in maintaining glycaemic control/glucose homeostasis include the liver, skeletal muscles, adipose tissue, the endocrine pancreas and the kidney. After consuming a meal, carbohydrates from food cross the intestinal wall to enter the blood stream. This results in a postprandial increase in the blood glucose level. A large fraction of the glucose arriving in the blood is taken up by the liver where it is stored in the form of glycogen (a glucose polymer), which can be re-converted to glucose later and released in the blood when needed to prevent hypoglycaemia. The storage and release of glucose is primarily regulated by two peptide hormones produced by the pancreas, namely insulin (which lowers blood glucose levels) and glucagon (which increases blood glucose levels).
- Insulin is produced by specialized cells in the pancreas known as β-cells, located within cell clusters known as Islets of Langerhans. Insulin is secreted into the blood and acts on various tissues in the body, including the liver, skeletal muscle and on fat cells known as adipocytes. Insulin secretion increases after meals, when there is a spike in circulating glucose, in order to lower blood glucose.
- Insulin stimulates glucose uptake by liver, muscle and fat cells. In the liver, insulin stimulates glucose conversion into (i) glycogen, in a process known as "glycogenesis"; and (ii) triglyceride fats, in a process known as "lipogenesis", which may be broken down by hydrolysis to produce energy. Insulin stimulates glycogenesis in skeletal muscle tissue and lipogenesis in adipose tissue (i.e. body fat), whereas the liver is capable of both glycogenesis and lipogenesis. The release of insulin therefore causes a reduction in the level of blood glucose.
- If blood glucose levels decrease, for example when glucose is being used for energy during exercise, the decrease in blood glucose stimulates the pancreas to produce glucagon in the α-cells of the Islets of Langerhans in the pancreas. Glucagon stimulates the conversion of stored glycogen into glucose in the liver for release into the blood, in a process known as "glycogenolysis". Glucagon also stimulates neoformation of glucose in the liver, a process called "gluconeogenesis".
- Diabetes mellitus ("diabetes") is a disease that affects the body's ability to use glucose. It is characterised by hyperglycaemia and disturbances in the metabolism of carbohydrates, proteins and fats which are caused by defects of insulin secretion, defects of insulin action, or both depending on the type of diabetes.
- Diabetic hyperglycaemia can cause microvascular and macrovascular complications, including damage to blood vessels over time, which in humans leads to complications in a range of organs including the eyes, kidney and heart causing blindness, renal failure and cardiovascular diseases, as well inducing peripheral nerve damage and neuropathies.
- There are two main types of diabetes: Type 1 diabetes ("T1DM"), or Insulin Dependent Diabetes Mellitus (IDDM); and Type 2 diabetes ("T2DM"), or Non-insulin Dependent Diabetes Mellitus (NIDDM).
- T1DM is an autoimmune disease characterized by a patient being unable to produce insulin. This results from an individual's immune system destroying the body's pancreatic β-cells, resulting in little or no endogenous insulin production. T1DM is a condition that commonly develops at a young age (although it can occur at any age) in which symptoms emerge relatively quickly in patients due to a decrease in the production of insulin below a critical level. Individuals with T1DM require subcutaneous injections of insulin in order to maintain glycaemic control and lower blood glucose levels.
- T2DM commonly happens later in life and develops during adulthood. It is a disease characterized by the gradual loss of insulin sensitivity (also termed insulin resistance), in various tissues, including the liver, muscle and adipose tissue, over time, as well as a reduction in insulin secretion by pancreatic β-cells. The gradual loss of insulin sensitivity leads to elevated blood glucose levels particularly after meals (impaired glucose tolerance), and then to an increase in fasting glucose levels. Because the body does not respond effectively to insulin, cells do not take up sufficient glucose from the bloodstream, resulting in high blood glucose levels. As a result, the body perceives a lack of glucose within cells and initiates several responses. It releases certain hormones that prompt the liver and muscles to break down stored glycogen and release glucose into the bloodstream, further increasing blood glucose levels.
- As a response to this hyperglycaemia, insulin secretion from β-cells initially increases in an attempt to compensate by increasing the blood glucose lowering effect. This eventually leads to β-cell dysfunction as the β-cells are unable to keep up with the increased demand for insulin. Combined with the reduced insulin sensitivity (insulin resistance) this eventually leads to persistent hyperglycaemia. This in turn exerts a deleterious effect on insulin secretion by impairing insulin gene transcription leading to a further decrease in the synthesis and secretion of insulin (glucose toxicity).
Treatment of T2DM
- The standard treatment for T2DM involved a stepwise progression from non-pharmacological measures (i.e. targeting a patient's lifestyle, for example, exercise and diet) towards the use of oral antidiabetic agents and finally insulin. Where there was inadequate control of glycaemia or inadequate relief of symptoms by diet or exercise, patients would be advised to start oral agent monotherapy.
- Various different classes of antidiabetic agents were available which worked by addressing (i) insulin resistance/sensitivity, (ii) β-cell dysfunction; (iii) carbohydrate digestion; or (iv) loss of β-cell mass.
- The following treatments fell into these classes:
i) Biguanides. Metformin was the only biguanide compound approved in the UK. It was introduced as a medication for diabetes in the UK in the early 1960s and had become an established, first line treatment for T2DM in the UK. The mechanism of action of metformin was not fully understood but was thought to affect insulin signalling, glucose uptake and metabolism, including to lower blood glucose levels by decreasing gluconeogenesis and glycogenesis, therefore lowering glucose production and improving insulin sensitivity in the liver.
ii) Thiazolidinediones ("TZDs"). TZDs were first introduced for treating T2DM in the 1990s and were approved for use in the UK. They belong to a class of drugs known as "PPAR agonists". The receptor that thiazolidinediones bind to is PPARγ, which is mostly expressed in adipose tissue, and activation was thought to affect the transcription of several genes involved in glucose and lipid metabolism. TZDs also affect insulin sensitivity. TZDs reduce insulin resistance in adipose tissue, muscle and the liver.
iii) Sulphonylureas. Developed in the 1950s and 1960s, sulfonylureas activate the cellular mechanism of insulin release from β-cells. The most recent class member (glimepiride) was authorised in the UK in 1995.
iv) Meglitinides. These compounds were developed in the late 1990s and 2000s and produce a rapid, short-lived stimulation of insulin secretion, allowing them to be taken before a meal to help prevent post-prandial hyperglycaemia. The mechanism of action of the meglitinides is similar to that of the sulphonylureas.
v) α-glucosidase inhibitors. These compounds act in the cells lining the intestine. Inhibiting α-glucosidase enzymes at the surface of these cells slows the breakdown of carbohydrates (including sucrose, starch and other complex sugars) into simple sugars, which reduces the intestinal absorption of glucose and therefore its blood glucose-raising effect. They were first developed in the 1970s, and the only approved α-glucosidase inhibitor in the UK was acarbose, which was introduced in the early 1990s.
vi) Insulin. Insulin replacement therapy is typically reserved for the later stages of T2DM once significant β-cell failure has occurred and oral agents will no longer exert sufficient effect. This required regular (daily or more frequent) injections and further lifestyle restrictions and was therefore generally undesirable unless other treatment options had been ruled out.
Emerging/potential treatments for T2DM
- There were several classes of emerging treatments which had either been: (1) reported in the literature and showing theoretical promise as pre-clinical agents; (2) entering clinical trials; or (3) recently becoming available for clinical use. These included (though various other targets were being looked into):
i) GLP-1 agonists. Glucagon-like peptide-1 ("GLP-1") is an endogenous metabolic hormone in the "incretin" family made by the small intestine. GLP-1's effects include stimulating insulin release and inhibiting glucagon secretion in the pancreas. It had been shown that injection of GLP-1 before meals substantially reduced post-prandial blood glucose concentrations, but GLP-1 is a peptide which is rapidly degraded. Therefore, various formulations were being investigated to address this issue. GLP-1 agonists were developed to act as exogenous compounds having the same anti-hyperglycaemic effects as GLP-1.
ii) DPP-4 inhibitors. They inhibit dipeptidyl peptidase 4 ("DPP-4"), an enzyme which is involved in glucose metabolism, including the breakdown of GLP-1. Inhibition of DPP-4 can therefore have a similar effect as GLP-1 agonists.
- Accurate measurement of glucose levels in blood or plasma is important to diagnose and assess the progress of diabetes. In humans, several methods of measuring blood or plasma glucose levels can be used. The oral glucose tolerance test was established as the most robust measure on which to diagnose diabetes. In that test, following an overnight fast, plasma glucose is drawn before, and at 30 minute intervals for 2 hours after, administration of a controlled dose of glucose.
- The presence of glucose in the urine (glucosuria) is a traditional indicator of diabetes, usually measured by testing urine with glucose-sensitive test strips to give a semi-quantitative assessment of glucose in urine.
Pre-diabetes
- Pre-diabetes was also known as impaired glucose tolerance and was a label given to patients who did not meet the diagnostic criteria for T2DM. Prof. Bailey agreed that it was not recognised as a separate disease or as suitable for pharmacological treatment. However, the evidence also established that the diagnostic cut-off was to some extent arbitrary, and textbook and review article authors were proposing that the diagnostic criteria should be adjusted to include pre-diabetes patients and/or that patients should be treated to prevent diabetes from occurring.
Assays
In vitro analysis
- Protocols for cloning genes and recombinant protein expression were well-established and frequently performed in the field. The Xenopus laevis expression system could be used to express membrane proteins. Other known cell lines commonly used to express proteins included Chinese Hamster ovary (CHO) and HEK 293 cells.
- In order to characterize a recombinant protein in vitro or test the effect of an inhibitor of a protein, after establishing a cell line and confirming expression of the protein, assays are used to assess its function.
- The effect of an inhibitory compound on the target can be measured in terms of IC50, which equates to the concentration that will inhibit the biological effect by 50%.
- It is most common to describe a substrate generating a positive response in terms of EC50 (i.e. the concentration that will produce 50% of the maximal biological effect), and an inhibitor of a biological process or component in terms of IC50. However, in practice these terms are often used interchangeably when describing the effect of an inhibitor.
- To calculate the IC50 for an inhibitor of a particular transporter protein, an assay is performed at a given substrate concentration and with the addition of a range of inhibitor concentrations up to a concentration that fully inhibits transport activity.
In vivo analysis
- Various animal models had been developed for testing agents with potential to treat T2DM and to study the biological processes underlying hyperglycaemia. These were regularly used in early research to test the viability of a new treatment in vivo. The most commonly studied animal models of T2DM were laboratory rodents (i.e. mice and rats), in which diabetes is often preceded and accompanied by obesity, although larger mammal models also existed but were used more rarely in the field.
- In such animals, features of T2DM are caused either by inherited genetic defects, environmental factors such as the diet and activity level of the animals, or by administering drugs to impair artificially either insulin production or insulin sensitivity. However, no single animal model provided an entirely accurate model of T2DM in humans. The most commonly used rodent models (which were used in diabetes drug development and research) included: the obese mouse (ob/ob), the diabetic mouse (db/db), the KK mouse, the streptozotocin-induced diabetic rat and the Zucker diabetic fatty rat.
In vitro / in vivo correlation
- There was a dispute about the extent to which the results of an in vitro assay allowed a prediction to be made of the results of an in vivo assay, though in the end it appeared to me that the difference between the experts was more one of language and attitude than substance. It was common ground that without a positive result in vitro no in vivo assay would be conducted (and it would be unethical to do so). Prof. Bailey agreed with Prof. Thorens that in vitro experiments were not directly predictive of how a compound would perform in vivo; he said they could provide some indication of how a compound may perform. For example, Prof. Bailey said, an inhibitor which works in vitro would be expected to have at least some degree of effect in vivo, though he accepted that the effect could be too low or there could be no effect at all. Prof. Thorens expressed it as "a hope" and said that some people were more optimistic than others about observing an effect in vivo given a positive result in vitro. Dr Edwards said that in vitro tests gave some idea of whether the compound is likely to work in vivo, though he said that in order to make a prediction from an in vitro result about likely efficacy in vivo, it would be necessary to know the in vitro / in vivo correlation. I return to this point below in the specific context of SGLT2 inhibitors.
- Glucose reabsorption occurs in both the intestine and the kidneys and is mediated by sodium glucose co-transporters ("SGLTs"). In the intestine, glucose transport is driven by the amount of sodium (Na+) present in the intestinal lumen.
- The kidney is made up of filtering units known as nephrons. The fluid that is filtered into the renal tubules (nephrons) is referred to as the filtrate. When blood passes through the kidney it is filtered of most small molecules, including glucose, which is then retrieved as the filtrate passes through the kidney, restoring glucose to the blood before it leaves the organ.
- The transport of Na+ ions and glucose molecules across the cell membrane is driven by an electrochemical driving force created by Na+ ions in the tubule moving into the cell. This is known as "secondary active transport".
- Glucose reabsorption takes place in a part of the nephron called the proximal convoluted tubule ("PCT") by the following steps:
i) SGLTs are expressed by the epithelial cells lining initial segments of the PCT and are located in the apical border of the cells which face into the lumen of the tubules. SGLT proteins transport Na+ ions and glucose molecules from the filtrate-containing tubule into the epithelial cells.
ii) Glucose is then transported out of the epithelial cells and into the interstitial compartment and blood by facilitated diffusion, via other types of glucose transporter proteins in the "GLUT" family (which are 'facilitative' glucose transporters that do not require Na+ ions for glucose transport).
iii) At the same time, Na+ ions are pumped out of the tubular epithelial cells and into the blood.
iv) Any glucose that is not reabsorbed from the glomerular filtrate in this way is ultimately excreted by the body in urine.
- In healthy individuals, the kidney will usually filter and reabsorb all of the available glucose in the blood. However, if not all of the glucose in the ultrafiltrate is reabsorbed in the kidney, either because blood glucose is too high or because the process of reabsorption is hampered, glucose is excreted in the urine (known as "glucosuria" or "glycosuria"). Glucosuria is commonly observed in individuals with diabetes, as a result of their elevated blood glucose levels.
- Two sodium-glucose co-transporter proteins had been isolated, cloned and characterized in humans, SGLT1 and SGLT2. SGLT1 was found to be expressed in both the kidney and intestine. SGLT2 was found to be expressed in the kidney but not the intestine. In addition to the SGLT proteins, several members of the GLUT family of proteins had been characterized, including GLUT1 and GLUT2 which were understood to be expressed in the kidneys.
- When the SGLTs in the intestine are congenitally defective, the resulting glucose-galactose malabsorption causes severe diarrhoea that may be fatal.
- Within the kidney, SGLT1 and SGLT2 can be distinguished by their location, their glucose transport capacity, and their relative affinities for glucose and sodium:
i) SGLT2 is highly expressed in the early segment of the PCT. It is a high capacity, low affinity co-transporter. This means that SGLT2 is able to transport large quantities of glucose but binds with low affinity. SGLT2 transports one Na+ ion per glucose molecule. In healthy individuals, reuptake of most glucose in the tubule is carried out by SGLT2 proteins in the early segment of the PCT.
ii) SGLT1 is expressed mostly in the late segments of the PCT. In terms of its physiological properties, it is the inverse of SGLT2, that is to say it is a low capacity, high affinity co-transporter. This means that SGLT1 is capable of transporting smaller quantities of glucose but with higher affinity. SGLT1 transports two Na+ ions per glucose molecule. In healthy individuals, the remaining glucose in the tubule is reabsorbed by SGLT1 proteins in the S2 and S3 segments of the PCT. SGLT1 is also known to be expressed in the intestines and was the transporter responsible for reabsorption in the intestines.
- Below is a diagram illustrating the functions of SGLT2 in the early PCT (top) and SLGT1 in the late PCT (below):
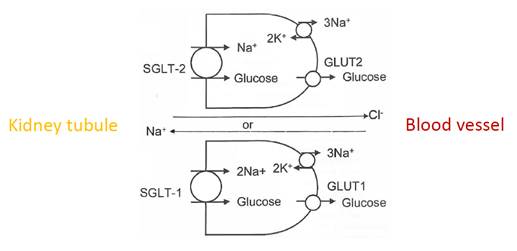
- It was known that inhibition of renal glucose reuptake would increase glucose excretion in the urine, therefore reducing blood glucose levels.
Phlorizin
- Phlorizin (sometimes referred to as phlorhizin or phloridzin) is a naturally occurring compound (derived from the bark of the apple tree (Malus domestica)) which competes with glucose for binding to the SGLTs.
- Phlorizin was discovered in the early 1800s and was later noted to produce glycosuria and reduce blood glucose levels. It was demonstrated in the 1960s that phlorizin inhibits renal glucose reabsorption, and the skilled biologist/ pharmacologist would be aware of studies using phlorizin, including to lower blood glucose in animal models. The skilled biologist/pharmacologist would (at least) assume that phlorizin inhibits both SGLT1 and SGLT2.
WO 128 and the Patent
- It is, of course, necessary to consider WO128 and the Patent separately when addressing the issues in this case. However, it is helpful at this stage to set out the common aspects of their disclosures and identify the ways in which their disclosures differ. Below, I have used the paragraph numbers in the Patent when addressing the common aspects.
- Both documents are entitled "C-aryl glucoside SGLT2 inhibitors and method" and in each case the "Field of the Invention" section (paragraph [0001]) explains that:
"The present invention relates to C-aryl glucosides which are inhibitors of sodium dependent glucose transporters found in the intestine and kidney (SGLT2) and to a method for treating diabetes, especially type II diabetes..."
The Background of the Invention
- The "Background of the Invention" section starts (in paragraph [0002]) by referring to the prevalence of T2DM (or NIDDM), the fact that it is characterised by hyperglycaemia due to excessive hepatic glucose production and peripheral insulin resistance, and that hyperglycaemia is considered to be the major risk factor for the development of diabetic complications. It continues:
"Normalization of plasma glucose in NIDDM patients would be predicted to improve insulin action, and to offset the development of diabetic complications. An inhibitor of the sodium-dependent glucose transporter SGLT2 in the kidney would be expected to aid in the normalization of plasma glucose levels, and perhaps body weight, by enhancing glucose excretion."
- It then explains (paragraph [0003]) that the development of novel, safe, orally-active antidiabetic agents is desirable to complement existing therapies and avoid their side effects.
- Paragraph [0004] starts:
"Hyperglycemia is a hallmark of type II diabetes (NIDDM); consistent control of plasma glucose levels in diabetes can offset the development of diabetic complications and beta cell failure seen in advanced disease. Plasma glucose is normally filtered in the kidney in the glomerulus and actively reabsorbed in the proximal tubule. SGLT2 appears to be the major transporter responsible for the reuptake of glucose at this site."
- It then refers to the effects of phlorizin:
"The SGLT specific inhibitor phlorizin or closely related analogs inhibit this reuptake process in diabetic rodents and dogs resulting in normalization of plasma glucose levels by promoting glucose excretion without hypoglycemic side effects."
- That is followed by a reference to a study using an unnamed SGLT2 inhibitor in a rat model:
"Long term (6 month) treatment of Zucker diabetic rats with an SGLT2 inhibitor has been reported to improve insulin response to glycemia, improve insulin sensitivity, and delay the onset of nephropathy and neuropathy in these animals, with no detectable pathology in the kidney and no electrolyte imbalance in plasma."
- Paragraph [0004] concludes as follows:
"Selective inhibition of SGLT2 in diabetic patients would be expected to normalize plasma glucose by enhancing the excretion of glucose in the urine, thereby improving insulin sensitivity, and delaying the development of diabetic complications."
- Paragraph [0005] picks up the point that was made early in paragraph [0004] (see paragraph 149 above):
"Ninety percent of glucose reuptake in the kidney occurs in the epithelial cells of the early S1 segment of the renal cortical proximal tubule, and SGLT2 is likely to be the major transporter responsible for this reuptake."
- That is followed by information about SGLT2 in support of that statement, and the paragraph concludes by saying:
"Inhibition of SGLT2 would be predicted to reduce plasma glucose levels via enhanced glucose excretion in diabetic patients."
- Paragraph [0006] contains information about SGLT1 and differences between SGLT1 and SGLT2 in terms of their amino acid sequence, location of expression, molar ratio of Na+ to glucose transported, Km values for glucose and the glucose analogue AMG, and substrate specificities.
- Paragraph [0007] returns to the effects of phlorizin:
"Administration of phlorizin, a specific inhibitor of SGLT activity, provided proof of concept in vivo by promoting glucose excretion, lowering fasting and fed plasma glucose, and promoting glucose utilization without hypoglycemic side effects in several diabetic rodent models and in one canine diabetes model. No adverse effects on plasma ion balance, renal function or renal morphology have been observed as a consequence of phlorizin treatment for as long as two weeks. In addition, no hypoglycemic or other adverse effects have been observed when phlorizin is administered to normal animals, despite the presence of glycosuria."
- It then refers to a study by Tanabe Seiyaku, which it was common ground would be understood to be the same study as that in Zucker diabetic rats referred to in paragraph [0004]:
"Administration of an inhibitor of renal SGLTs for a 6-month period (Tanabe Seiyaku) was reported to improve fasting and fed plasma glucose, improve insulin secretion and utilization in obese NIDDM rat models, and offset the development of nephropathy and neuropathy in the absence of hypoglycemic or renal side effects."
- Paragraph [0008] gives two reasons why phlorizin is unattractive as an oral drug, namely that it a nonspecific SGLT1/SGLT2 inhibitor, and that it is hydrolysed in the gut to its aglycone phloretin, which is a potent inhibitor of facilitated glucose transport. It then explains why those are regarded as problems:
"Concurrent inhibition of facilitative glucose transporters (GLUTs) is undesirable since such inhibitors would be predicted to exacerbate peripheral insulin resistance as well as promote hypoglycemia in the CNS. Inhibition of SGLT1 could also have serious adverse consequences as is illustrated by the hereditary syndrome glucose/galactose malabsorption (GGM), in which mutations in the SGLT1 cotransporter result in impaired glucose uptake in the intestine, and life-threatening diarrhea and dehydration."
The paragraph ends by pointing out that the biochemical and sequence differences between SGLT2 and SGLT1 (see paragraph 155 above) allow for the identification of selective SGLT2 inhibitors.
- Paragraph [0010] then suggests that reducing renal re-uptake of glucose may have minimal long term adverse consequences, based on experience in people with familial glycosuria:
"The familial glycosuria syndromes are conditions in which intestinal glucose transport, and renal transport of other ions and amino acids, are normal. Familial glycosuria patients appear to develop normally, have normal plasma glucose levels, and appear to suffer no major health deficits as a consequence of their disorder, despite sometimes quite high (110-114 g/daily) levels of glucose excreted. The major symptoms evident in these patients include polyphagia, polyuria and polydipsia, and the kidneys appear to be normal in structure and function. Thus, from the evidence available thus far, defects in renal reuptake of glucose appear to have minimal long term negative consequences in otherwise normal individuals."
- Up to this point, the descriptions of WO 128 and the Patent are identical. Each document then turns to refer to various prior art documents.
- WO 128 says that "the following references disclose O-aryl glucosides SGLT2 inhibitors for treating diabetes". It then refers to a number of European and Japanese patent applications (the first of which is attributed to Tanabe Seiyaku). It then says that "other disclosures and publications which disclose SGLT2 inhibitors include the following"; it then cites a paper by Tsujihara et al. (Chem. Pharm. Bull., 1996), two papers by Hongu et al. (Chem. Pharm. Bull., 1998) and a paper by Oku et al. (Diabetes, 1999). Finally it refers to a patent application (WO 98/31697) which it says discloses compounds according to a Markush formula (of C-aryl glucosides) which are disclosed for use in the treatment of a wide range of diseases and conditions including diabetes.
- The Patent instead says that "the following references disclose C-aryl glucosides SGLT2 inhibitors for treating diabetes". It then refers to WO 128, which it says discloses compounds of a particular structure (formula I in WO 128 - see paragraph 163 below) which it says are "reported to be inhibitors of the SGLT2 transporter and consequently represent a mode for treatment of diabetes and complications thereof" and to WO 98/31697 (in the same terms as does WO 128).
The Description of the Invention
-
In WO 128, the "Description of the Invention" section starts by explaining that the invention provides C-aryl glucosides of formula I:
- It then proceeds to explain the options for R1, R2, R2a, R3, R4 and A. It is not necessary to set them out - suffice it to say that it was common ground that the number of compounds encompassed by formula I is vast.
- WO 128 then asserts:
"The compounds of formula I possess activity as inhibitors of the sodium dependent glucose transporters found in the intestine and kidney of mammals and are useful in the treatment of diabetes and...complications of diabetes..."
- It then says that the invention includes a method for treating or delaying the progression or onset of diabetes, including complications of diabetes, and various related diseases, involving the administration of a therapeutically effective amount of compound of formula I to a human patient in need of treatment.
- WO 128 goes on to state that compounds of formula IA are preferred. In formula IA the options for R1, R2, R2a, R3, R4 and A are reduced compared to formula I, but the number of compounds encompassed is still vast.
- It then says that "most preferred" are compounds of formula IB:

- In formula IB, A has been limited to -CH2- at the meta position of the first phenyl ring and R2, R2a and R3 have been limited to hydrogen. Further, the options for R1 are limited to hydrogen, halogen or lower alkyl at the para position of the first phenyl ring and those for R4 are limited to lower alkyl, -OR5a, -OCHF2 or -SR5e (where R5a and R5e are both alkyl) at the para position of the second phenyl ring. However, it was common ground that the number of compounds encompassed by formula IB was still in the millions.
- By contrast, in the Patent, the "Description of the Invention" section explains in [0013] that the invention provides a C-aryl glycoside compound of formula I:
- That structure is that of the molecule now known as dapagliflozin. The Patent adds that it includes "pharmaceutically acceptable salts thereof, all stereoisomers thereof, and all prodrug esters thereof".
- At paragraph [0014] the Patent then makes the same assertion as WO 128 (see paragraph 165 above):
"The compound of formula I possesses activity as inhibitors [sic] of the sodium dependent glucose transporters found in the intestine and kidney of mammals and is useful in the treatment of diabetes and...complications of diabetes..."
- Like WO 128 (see paragraph 166 above), it then says that the invention includes a method for treating or delaying the progression or onset of diabetes, including complications of diabetes, and various related diseases, involving the administration of a therapeutically effective amount of compound of formula I to a human patient in need of treatment.
The Detailed Description of the Invention
- In both WO 128 and the Patent, the "Detailed Description of the Invention" sets out reaction schemes for the preparation of compounds of the invention. It was common ground that the chemistry described was standard and well within the capabilities of the skilled medicinal chemist. That is followed by a number of definitions, as well as explanations that salts or stereoisomers of compounds of the invention can be used (in the Patent these are in paragraphs [0034]-[0049]). Each document then contains a number of paragraphs (in essentially identical form) explaining that the compounds of the invention can be used in combination with other types of anti-diabetic agent and/or other types of therapeutic agent, giving many examples. It should be noted that at various points the compounds of the invention are described as SGLT2 inhibitors (see e.g. paragraphs [0051]-[0053], [0074] and [0102] in the Patent).
The Assay for SGLT2 Activity
- Each document then states that "SGLT2 inhibitor activity of the compounds of the invention may be determined by use of an assay system as set out below" (this is paragraph [0114] in the Patent). The following description of the assay is then provided (at paragraph [0115] in the Patent):
"The mRNA sequence for human SGLT2 (GenBank #M95549) was cloned by reverse-transcription and amplification from human kidney mRNA, using standard molecular biology techniques. The cDNA sequence was stably transfected into CHO cells, and clones were assayed for SGLT2 activity essentially as described in Ryan et al. (1994). Evaluation of inhibition of SGLT2 activity in a clonally selected cell line was performed essentially as described in Ryan et al., with the following modifications. Cells were grown in 96-well plates for 2-4 days to 75,000 or 30,000 cells per well in F-12 nutrient mixture (Ham's F-12), 10% fetal bovine serum, 300 ug/ml Geneticin and penicillin-streptomycin. At confluence, cells were washed twice with 10 mM Hepes/Tris, pH 7.4, 137 mM N-methyl-D-glucamine, 5.4 mM KCl, 2.8 mM CaCl2, 1.2 mM MgSO4. Cells then were incubated with 10 mM [14C]AMG, and 10 mM inhibitor (final DMSO =0.5%) in 10 mM Hepes/Tris, pH 7.4, 137 mM NaCl, 5.4 mM KCl, 2.8 mM CaCl2, 1.2 mM MgSO4 at 37oC for 1.5 hr. Uptake assays were quenched with ice cold 1X PBS containing 0.5 mM phlorizin, and cells were then lysed with 0.1% NaOH. After addition of MicroScint scintillation fluid, the cells were allowed to shake for 1 hour, and then [14C]AMG was quantitated on a TopCount scintillation counter. Controls were performed with and without NaCl. For determination of EC50 values, 10 inhibitor concentrations were used over 2 log intervals in the appropriate response range, and triplicate plates were averaged across plates."
The reference for the cited Ryan et al. (1994) paper is then provided.
The Examples
- Each document then says: "The following Working Examples represent preferred embodiments of the present invention" (in the Patent, this is paragraph [0117]).
- In WO 128 there follow 15 Examples in which a full methodology is given, along with the masses of compounds produced and results of HPLC, NMR and molecular mass analyses. There are then two tables, containing a further 65 examples, in which the methodology is indicated by reference to one of Examples 1-15 and the results of molecular mass analysis is stated. It was common ground that the medicinal chemist would understand that all the compounds of the 80 Examples had in fact been synthesised. The identities of the A linker and the substituents on the phenyl rings in the compounds of the 80 Examples were set out by Prof. Potter in a table exhibited to his first report. None of the exemplified compounds is dapagliflozin (the closest is Example 12, which differs in that the group at the R4 position is -OMe whereas it is -OEt in dapagliflozin).
- WO 128 does not contain any results of assays conducted using any of the compounds of the 80 Examples (or any other compounds), whether using the assay described or otherwise.
- In the Patent, paragraph [0117] is followed by only one Example in which the preparation of the compound of formula I is described. Full methodologies are provided, together with the mass of the product produced (about 20 g) and results of analyses of the same types as in Examples 1-15 of WO 128.
- As with WO 128, no results of any assay (whether that described or otherwise) are provided.
The Claims
- The claims of WO 128 track the description (thus, for example, claim 1 corresponds to formula I, claim 14 corresponds to formula IB, claims 10-13 together correspond to the Examples and claims 26 and 27 are claims to methods of treating, inter alia, diabetes using compounds of formula I or formula IB respectively).
- The claims of the Patent which were defended by AZ are claims 1, 2, 14 and 15. AZ has applied to amend claim 14 to delete all the diseases originally listed save for diabetes. The only ground of opposition to that amendment raised by the Claimants is that it does not cure the invalidity of the Patent. The Comptroller raised no objections to the amendment. Accordingly, it is only necessary to consider the amended version of claim 14.
- Claim 1 is to "A compound having the structure [of dapagliflozin] or a pharmaceutically acceptable salt, a stereoisomer thereof, or a prodrug ester thereof". Claim 2 is to "The compound as defined in claim 1 having the structure [of dapagliflozin]". Claim 14 as amended is to the "Use, in the manufacture of a medicament for treating or delaying the progression or onset of diabetes, of a compound as defined in claim 1". Claim 15 is to "The use as defined in claim 14 where the SGLT2 inhibitor compound has the structure [of dapagliflozin]".
- In the end the Claimants did not press any point about the inclusion in claim 1 (and claim 14) of pharmaceutically acceptable salts, stereoisomers and prodrug esters of dapagliflozin. Nor did AZ contend that claims 14 and 15 could be valid if claims 1 and 2 were invalid. However, the Claimants did maintain a case that claims 14 and 15 were invalid even if claims 1 and 2 were valid. The upshot is that the issues can be decided by reference only to claim 2 (to dapagliflozin itself) and claim 15 (in summary, to the use of dapagliflozin in the manufacture of a medicament for treating or delaying the progression or onset of diabetes).
The Skilled TeaM and The DISPUTED CGK
- There was a lively debate between the parties as to the makeup of the skilled team and, consequentially, the extent of its CGK relating to SGLT2 inhibitors as potential treatments for diabetes. The Claimants were keen to be able to say that much of paragraphs [0002]-[0010] of the Patent was CGK, while AZ was keen to be able to say the opposite. However, ultimately this dispute has little, if any, effect on the overall conclusions in this case, for the following reasons.
- First, it was common ground that when assessing whether a compound has plausible utility in treatment of a disease, it does not matter whether the mechanism relied on as linking the compound's activity with an effect on the disease state is disclosed in the patent for the first time or was known from the prior art (see Salk and Warner-Lambert). So for that purpose it is immaterial whether most of paragraphs [0002]-[0010] was CGK. It should also be noted that neither party contended that there was any CGK relevant to SGLT2 inhibitors as potential treatments for diabetes which was not in paragraphs [0002]-[0010].
- Secondly, as explained above, the material in paragraphs [0002]-[0010] of the Patent is also present in WO 128. So even if those paragraphs brought together material which was not CGK, that is not part of the technical contribution to the art made by the Patent.
The skilled team
- As I have indicated, the difference between the parties as to the makeup of the skilled team related to the skilled biologist/pharmacologist. In essence, the Claimants contended that the skilled biologist/pharmacologist would have a specialism in glucose transporter proteins and diabetes, whereas AZ contended that they would be focused on diabetes, but not on glucose transporter proteins.
- AZ referred me to the analyses by Birss J in Illumina HC at [58]-[70] and by Meade J in Alcon v Actavis [2021] EWHC 1026 (Pat) at [30]-[31], as well as to the way in which the principles were applied to the facts in Illumina HC at [73]-[96] and by Meade J in Teva v Astellas [2022] EWHC 1316 (Pat) at [36]-[48]. The Claimants referred me to the review of the authorities by Mellor J in Alcon v AMO [2022] EWHC 966 (Pat) at [210]-[215], including the summary of principles by Henry Carr J in Garmin v Philips [2019] EWHC 107 (Pat) at [85] and the analysis by Kitchin LJ in Medimmune v Novartis [2012] EWCA Civ 1234 at [73]-[76].
- As Henry Carr J said in Garmin at [85], a patent specification is addressed to those likely to have a real and practical interest in the subject matter of the invention (which includes making it as well as putting it into practice). However, that does not greatly assist in deciding on the appropriate skilled person in a case where the dispute is about whether the field is a narrower one or a broader one which encompasses the narrower one.
- In Medimmune the question of whether the correct field was a narrower one or a broader one encompassing the narrower one was answered by reference to the reality of the position at the time and the skills of real research teams in the art. Kitchin LJ endorsed the approach of asking what problem the invention aimed to solve and in what art that problem lay.
- In Illumina HC Birss J was considering the identity of the skilled person for the purpose of obviousness in a case where that differed from the skilled person for the purpose of sufficiency (a Schlumberger-type case). He reviewed a number of cases concerned with the identification of the skilled person, including Garmin, MedImmune and Schlumberger and emphasised the unfairness that could arise, either to the patentee or the public, in defining the skilled person too narrowly or too broadly. He held at [68] that in a Schlumberger-type case the approach to take was:
"(i) To start by asking what problem does the invention aim to solve?
(ii) That leads one to consider what the established field which existed was, in which the problem in fact can be located.
(iii) It is the notional person in that established field which is the relevant team making up the person skilled in the art."
- In Alcon v Actavis Meade J referred to the analysis by Birss J in Illumina HC at [58]-[70] and said at 31:
"I intend to apply that approach. I take particular note of:
i) The requirements not to be unfair to the patentee by allowing an artificially narrow definition, or unfair to the public (and the defendant) by going so broad as to "dilute" the CGK. Thus, as Counsel for Alcon accepted, there is an element of value judgment in the assessment.
ii) The fact that I must consider the real situation at the priority date, and in particular what teams existed.
iii) The need to look for an "established field", which might be a research field or a field of manufacture.
iv) The starting point is the identification of the problem that the invention aims to solve."
- I agree with AZ that a team interested in diabetes treatments generally would have a practical interest in an SGLT2 inhibitor which was said to be useful to treat diabetes. But that does not help to resolve the question of whether the skilled team is properly to be set so broadly or whether it should be defined more narrowly.
- I need to start by identifying the problem that the invention aims to solve. In my judgment there can only be one answer to that question. The Patent recognises in the Background of the Invention section that there has been prior work on SGLT2 inhibitors with the aim of treating diabetes. The problem that the invention aims to solve is to provide a further SGLT2 inhibitor for treating diabetes. In other words, the invention does not aim to provide a new mechanism for the treatment of diabetes, but a new compound of a type previously proposed for that purpose, namely an SGLT2 inhibitor.
- Next I need to consider what established field that problem lay in. Was the field that of treatments for diabetes generally, as AZ contends, or a narrower field with a focus on glucose transporter proteins as potential targets for diabetes therapy? Was the latter an established field?
- It was common ground that there were academic groups (including that of Prof. Thorens) doing basic research on glucose transporter proteins, but they were not working on those proteins as potential targets for diabetes treatment. AZ relied on the fact that publications by such groups did not refer to SGLT2 inhibitors as potential treatments for diabetes. But that is not surprising, if that was not the focus of their work.
- The Claimants relied on evidence showing that there were a number of pharmaceutical companies actively seeking SGLT2 inhibitors as potential diabetes treatments. Apart from BMS itself, work had been done by Tanabe Seiyaku in that area. Tanabe Seiyaku had published patent applications and a number of papers, namely the four cited in WO 128 and Arakawa et al. (Br. J. Pharmacol., 2001). Further, as shown by the 2005 review article by Handlon, by the priority date patent applications for SGLT2 inhibitors as potential antidiabetic agents from the Japanese companies Kissei, Kotobuki and Ajinomoto had been published; moreover, by the end of 2002 Yamanouchi, Taisho and Aventis had also filed such patent applications (and the evidence was that the work contained in them was likely to have been started before the priority date). It appeared from a paper published in 2000 that Abbott also had an interest in SGLT2 inhibitors as potential diabetes treatments.
- AZ's response to this was to say that this work was being carried out "covertly" without others being aware of it. The factual basis for this assertion was unclear. Patent applications and papers had been published, as I have said. Further, it is clear that BMS was aware of the Tanabe Seiyaku work, as were Abbott and Chiron (it was referred to in a 2001 review article by Chiron authors). But in any event, I cannot see why, if the work being done in an area would otherwise lead to the conclusion that there was an established field, it would matter that those active in the area were unaware of the activities of the others.
- AZ also sought to draw parallels with the way in which the legal principles were applied to the facts in Medimmune, Illumina HC and Teva v Astellas, suggesting that those cases showed that there needed to be more public activity, in the form of published papers and the like, before a field could be regarded as an established one. While those cases are helpful in showing the kinds of factors that can be taken into account in determining whether a field is an established one, they do not purport to set a threshold, and each case must turn on its own facts.
- AZ drew attention to the fact that no SGLT2 inhibitors had been licensed as drugs by the priority date, and to the fact that diabetes textbooks and review articles (save for the Chiron one) at around the priority date did not identify SGLT2 inhibition as a potential approach to treatment of diabetes. I agree that this shows that the potential for SGLT2 inhibitors as treatments for diabetes had not become generally known and accepted amongst those interested in diabetes treatments generally. But that begs the question of whether there was an established field which would have been aware of such matters.
- In my judgment, by the priority date there was sufficient activity in the area of SGLT2 inhibitors as potential treatments for diabetes for that to have become an established field. I agree with the Claimants that those in that field would also have had a focus on other glucose transporter proteins, as part of the glucose transport system which included SGLT2.
- Finally, I ask myself whether defining the field in the narrower way contended for by the Claimants rather than the broader way contended for by AZ is unfair to the patentee. I do not see that it is. The Patent proceeds on the basis that the potential utility of SGLT2 inhibitors in the treatment of diabetes is known and that its contribution lies in the provision of a further SGLT2 inhibitor for that purpose. I cannot see what is unfair about defining the skilled person as someone who is aware of the potential utility of SGLT2 inhibitors in the treatment of diabetes.
- Further, it is notable that when Prof. Bailey was asked to set out the CGK of his pharmacologist, who was a diabetes generalist, he did not include anything about glucose transporter proteins or the mechanism of glucose reabsorption because he did not consider that they would have been the focus of his pharmacologist. He was only prompted to include a discussion of those matters and of phlorizin in his first report (a discussion which underlay the agreed CGK set out in paragraphs 133-144 above) by reading WO 128 and the Patent. The fact that the problem addressed by the Patent requires the explanation of background material which Prof. Bailey had not initially regarded as being relevant CGK rather suggests that defining the skilled pharmacologist as he had done involves viewing matters at too high a level of abstraction, and that to do so could be unfair to the public.
- Similarly, Prof. Potter explained that the strategic decision of identifying the drug target (in this case SGLT2) would come from the senior leadership team, who would instruct the medicinal chemist to work on a particular drug target. Dr Edwards explained that the skilled biologist in a drug discovery team would have a background in the biology of the target and an understanding of relevant assays. In a case where the problem which the Patent seeks to solve is the provision of a further SGLT2 inhibitor, rather than the identification of a new target, it appears wrong for the biologist/pharmacologist on the skilled team not to have a background in the biology of the target but instead to be a diabetes generalist. Again, that could be unfair to the public.
The disputed CGK
- The dispute over the CGK (apart from the aspects I have addressed above which did not depend on the identity of the skilled team) concerned (i) the extent of the skilled team's knowledge relating to glucose transporter proteins (including GLUTs and SGLTs) and phlorizin, and (ii) whether the skilled team considered SGLTs, particularly SGLT2, to be potential targets for treatment of diabetes at the priority date.
- It is important to recall that there was a degree of agreement about the CGK in relation to glucose transport and reabsorption, SGLTs and phlorizin (see paragraphs 133-144 above). In particular, it was common ground that the role of SGLT2 in glucose reuptake in the kidney was CGK, that it was CGK that inhibition of renal glucose reuptake would increase glucose excretion in the urine so reducing blood glucose levels, and that it was CGK that phlorizin inhibited renal glucose reabsorption and had been used to lower blood glucose in animal models. It was also agreed (though not stated in the agreed CGK document) that the skilled team would have known that they could look up the structure of phlorizin (e.g. in the Merck Index).
- It became clear during the evidence that the dispute between the parties as to this aspect of the CGK was intertwined with, and had not really been distinguished from, the dispute as to the identity of the skilled team. At the close of the evidence I therefore asked the parties to address in their closing submissions what they said was the difference in the CGK which flowed from a decision either way on the skilled team (and what impact, if any, that had on the issues in the case). Despite that, the parties did not provide much clarity about their positions on that question.
- In my judgment it follows from my conclusion as to the identity of the skilled team that the skilled team would have considered SGLT2 inhibitors to have potential for the treatment of diabetes. When that is combined with the agreed CGK (see paragraph 207 above) then I could not detect much residual difference between the parties as to the CGK of that skilled team.
- There was a dispute about whether the skilled team would have known that phlorizin was not suitable for use in humans because of its propensity to hydrolyse. In my judgment a skilled team as I have defined it, and with the agreed CGK about phlorizin, would be bound also to be aware of that, and such a conclusion was supported by the evidence of Prof. Thorens and Prof. Bailey, taken in the round.
- There was also a dispute about the extent to which the Tanabe Seiyaku work would have been CGK. In his report, Prof. Thorens had said that the Tanabe Seiyaku work referred to in paragraphs [0004] and [0007] of the Patent (paragraphs 151 and 157 above) would not have been CGK. In his oral evidence he suggested that while the skilled biologist would not have been aware of the 6 month rat study referred to in the Patent, the skilled biologist would have read Oku et al. (Diabetes, 1999) and been aware that the Tanabe Seiyaku compound T-1095a had been shown to be able to control hyperglycaemia in an animal model and was proposed as a treatment for diabetes. It was then put to Prof. Bailey that the skilled pharmacologist would have come across Oku et al. by scanning the contents list of Diabetes and also Arakawa et al. (Br. J. Pharmacol., 2001) in a similar manner. However, ultimately all that the Claimants said would be CGK as a result was that there was work being done on SGLT2 inhibitors to treat diabetes. Prof. Bailey agreed that the skilled team would have had that level of awareness, and I accept that.
- However, neither party submitted that the skilled team would have been aware, as part of their CGK, of the detail of the work that had been done. For example, ultimately neither party submitted that it would have been CGK that Tanabe Seiyaku was developing a compound called T-1095a, nor what its structure was, nor what results it had yielded in in vitro or in vivo models, nor what medicinal chemistry and SAR work had been done to arrive at T-1095a.
- I should also record what was not said to be CGK, by either party, about the mechanism proposed in the Patent for treatment of diabetes by SGLT2 inhibition. It will be recalled that paragraph [0002] of the Patent says:
"Normalization of plasma glucose in NIDDM patients would be predicted to improve insulin action, and to offset the development of diabetic complications. An inhibitor of the sodium-dependent glucose transporter SGLT2 in the kidney would be expected to aid in the normalization of plasma glucose levels, and perhaps body weight, by enhancing glucose excretion."
Similarly, paragraph [0004] says:
"Selective inhibition of SGLT2 in diabetic patients would be expected to normalize plasma glucose by enhancing the excretion of glucose in the urine, thereby improving insulin sensitivity, and delaying the development of diabetic complications."
- Prof. Thorens and Prof. Bailey agreed that these statements (and those in paragraphs [0004] and [0007] regarding the effects of phlorizin in animal models) would have been CGK, at least to the skilled team as I have defined it. However, neither party suggested that the skilled team would have been aware of the degree of SGLT2 inhibition that would need to be achieved to affect blood or plasma glucose levels or treat diabetes, or the potency of an SGLT2 inhibitor that would be needed to achieve that.
- I referred to the general position about potency requirements in paragraph 102 above. Prof. Bailey said that potencies in the low nanomolar range or better were not essential for every drug, and that the necessary potency depended on the target and the substrate. He said that compounds could be very effective with potencies in the micromolar range. He gave the example of metformin which he said had been shown to be effective in vitro in the micromolar concentration range and was administered at a dose of 500 mg per tablet. However, metformin operated by a different mechanism (see paragraph 120(i) above) and the evidence did not establish that efficacy at micromolar concentrations is the same as a micromolar EC50. In any event, Prof. Bailey agreed that a compound with a millimolar EC50 would not be regarded as acceptable.
- AZ submitted that the CGK was that potency was not particularly important for diabetes drugs. I do not agree that the evidence established that. The only basis for that submission was metformin (which as I have said operated by a different mechanism to SGLT2 inhibition) and phlorizin (which was not a diabetes drug; in any event neither party suggested that the IC50 for phlorizin was part of the CGK).
- Prof. Bailey suggested that a drug with a lower potency for SGLT2 but a higher "on" time (i.e. one which remains bound to SGLT2 for a longer period) could be as effective as one with a higher potency but a lower "on" time. However, he did not suggest that that had been established experimentally, let alone that the result formed part of the CGK. He also explained that it was not known what (if any) degree of selectivity for SGLT2 as compared to SGLT1 was required for efficacy. Moreover, he did not suggest that an in vitro / in vivo correlation had been established for SGLT2 inhibitors; neither party suggested that there was any particular reason to think that there would be a good, or poor, correlation between in vitro and in vivo activities for SGLT2 inhibitors.
- Overall, there was nothing in the CGK about SGLT2 inhibitors to alter the general position about potency requirements referred to in paragraph 102 above, or the general position about in vitro / in vivo correlation referred to in paragraph 132 above. The skilled team would be aware that the potency of a compound as an SGLT2 inhibitor in vitro would be important in determining its prospects of being effective in reducing blood / plasma glucose and treating diabetes. They would recognise that a compound with a millimolar EC50 would not be a credible candidate. The lower the EC50 the more likely it was that a compound would have sufficient efficacy in vivo to be useful in reducing blood / plasma glucose and treating diabetes. Ideally a compound for treating diabetes would have a low nanomolar EC50 or better, but the skilled team would recognise that a compound with a high nanomolar EC50 might prove to be useful. Doing the best I can with the limited evidence there was (for the reasons explained below), I agree with the Claimants' submission that someone seeking an experimental tool for lowering blood / plasma glucose would have wanted something with a comparable IC50 to phlorizin (though, as I have said, that value was not part of the CGK).
Plausibility
- In the present case the first Fibrogen step causes no difficulty. Claim 2 is limited to a single compound - dapagliflozin - while claim 15 is limited to the use of that compound in the manufacture of a medicament for treating or delaying the progression or onset of diabetes.
The second Fibrogen step
- Nor is there any difficulty about the second Fibrogen step in the case of claim 15. "Working", for the purpose of that claim, is treating or delaying the progression or onset of diabetes. That is the relevant technical effect for the purpose of claim 15.
- The second Fibrogen step is more difficult in the case of claim 2. The Claimants served a Part 18 request asking AZ to identify the technical contribution(s) on which it relied. AZ's response was that it relied on the following:
"(i) dapagliflozin and/or that dapagliflozin possesses activity as an SGLT2 inhibitor and is useful in the treatment and/or delayed progression of diabetes...;
(ii) dapagliflozin is a selective SGLT2 inhibitor in vitro and/or it reduces plasma glucose and/or blood glucose in vivo;
(iii) dapagliflozin is a more selective SGLT2 inhibitor in vitro than the compound in Example 12 of WO 128 and/or it reduces plasma glucose and/or blood glucose in vivo more effectively than the compound in Example 12 of WO 128;
(iv) dapagliflozin has a comparable in vivo efficacy to the compound in Example 10 of WO 128 but the compound in Example 10 of WO 128 cannot be used as a therapeutic agent due to stability issues."
- However, AZ did not advance a case at trial that dapagliflozin per se was a technical contribution (i.e. that a technical contribution lay in the provision of the compound per se without any useful activity). Nor did it rely on a technical contribution consisting of dapagliflozin being a selective SGLT2 inhibitor. Finally, AZ did not rely at trial on alleged technical contributions (iii) or (iv).
- In its opening skeleton, AZ advanced two technical contributions - that dapagliflozin was an SGLT2 inhibitor, and that it lowered blood glucose and so was useful in the treatment or delay of the progress or onset of diabetes. When I asked, during AZ's opening, whether it relied on any utility of dapagliflozin as an SGLT2 inhibitor other than in treatment etc. of diabetes, AZ said that it relied on utility as an experimental tool or to lower blood glucose. By the time of closing, that had been refined to utility as an experimental tool to lower blood glucose.
- In their reply closing speech, the Claimants took a pleading point, saying that utility as an experimental tool to lower blood glucose had not been pleaded. In my view, the time at which to take any point about utility as an experimental tool not being pleaded was when the issue was raised in opening, before the parties addressed the topic (albeit briefly) during the evidence. In any event, I do not think the pleading point has any merit. AZ was asked to identify the technical contributions on which it relied. Amongst the technical contributions it identified was the ability of dapagliflozin, as an SGLT2 inhibitor, to reduce blood (or plasma) glucose in vivo. The Claimants could have asked AZ whether it relied on any utility for such a technical contribution other than in the treatment etc. of diabetes, but they did not do so. I cannot see any reason to prevent AZ from relying on the utility of a technical contribution which it had pleaded in the form of an experimental tool, especially in the absence of any objection by the Claimants at the outset of the trial. However, the fact that utility as an experimental tool was raised only during AZ's opening meant that there was no evidence to support that submission in AZ's experts' reports (or to address it in the Claimants' expert reports) and there was only very limited oral evidence directed to the topic. I have had to do the best I can with that limited evidence.
- It is necessary to consider whether a technical contribution of dapagliflozin as an SGLT2 inhibitor which lowers blood glucose in vivo and so has utility as an experimental tool is a contribution of sufficient significance having regard to what Meade J said in Apixaban HC and Gilead v NuCana (see paragraph 31 above). In my view, it is. This is a case where the utility relied on is said to be provision of a compound which lowers blood glucose in vivo such that it can be used as a tool to conduct experiments, as phlorizin was. It is not a case where the only utility relied on is to provide a compound's own activity in an assay, which information can then be used to determine an SAR or the like.
- However, it is important to note that it was not suggested by AZ that utility as an experimental tool could arise from any level of SGLT2 inhibition in vitro or reduction in blood glucose in vivo (just as it was not said that utility as a treatment etc. for diabetes could arise from any level of SGLT2 inhibition in vitro or reduction in blood glucose in vivo). If AZ had contended that a technical contribution could lie in dapagliflozin being an SGLT2 inhibitor, even if the level of inhibition it produced was so low as to be of no practical utility, then I would have rejected that for the same reasons as those given by Meade J in Apixaban HC, i.e. that there is no technical contribution in a uselessly low degree of activity.
- It is also necessary to consider whether the technical contributions relied on by AZ have a basis in the specification of the Patent (see Meade J in Apixaban HC quoted in paragraph 30 above). Obviously the Patent asserts that dapagliflozin will treat etc. diabetes, and the Claimants did not contend otherwise. The dispute was about the alternative technical contribution contended for by AZ. The Patent clearly contemplates that an SGLT2 inhibitor such as dapagliflozin will reduce blood or plasma glucose, which is why it is said that dapagliflozin will be useful for treating diabetes - see for example the passages from paragraphs [0002] and [0004] quoted in paragraphs 147 and 152 above. It also recognises, by its references to phlorizin in paragraphs [0004], [0007] and [0008], that a compound that reduces blood or plasma glucose may be useful even if it is not attractive as a treatment for diabetes. While the Patent never in terms asserts that dapagliflozin will be useful (like phlorizin) as an experimental tool because of its ability to lower blood or plasma glucose, in my judgment there is sufficient in the specification to provide a basis for the alternative technical contribution relied on by AZ.
- The other issue which needs to be addressed at this point is whether the Patent is to be understood as asserting that, in order to be useful in treatment of diabetes, an SGLT2 inhibitor needs to be a selective SGLT2 inhibitor, i.e. selective for SGLT2 over SGLT1. The Claimants pointed to the sentence at the end of paragraph [0004] which said that "Selective inhibition of SGLT2 in diabetic patients would be expected to normalize plasma glucose...". However, the sentence at the end of paragraph [0002] says merely that "An inhibitor of...SGLT2 in the kidney would be expected to aid in the normalization of plasma glucose...", with no reference to selectivity. More importantly, the Claimants relied on paragraph [0008] which says, so far as relevant:
"Phlorizin itself is unattractive as an oral drug since it is a nonspecific SGLT1/SGLT2 inhibitor.... Inhibition of SGLT1 could also have serious adverse consequences as is illustrated by the hereditary syndrome glucose/galactose malabsorption (GGM), in which mutations in the SGLT1 cotransporter result in impaired glucose uptake in the intestine, and life-threatening diarrhea and dehydration. The biochemical differences between SGLT2 and SGLT1, as well as the degree of sequence divergence between them, allow for identification of selective SGLT2 inhibitors."
- The first point to make is that the Patent at no point asserts that dapagliflozin is selective for SGLT2 over SGLT1. It merely asserts that it is an SGLT2 inhibitor. Indeed if anything at points it appears to suggest that it also inhibits SGLT1, because it says that it inhibits SGLTs found in the intestine (see paragraphs [0001] and [0014]). It is no doubt for that reason that AZ abandoned its pleaded case that one of its technical contributions was that dapagliflozin was a selective SGLT2 inhibitor. It would be odd for the Patent to be read as saying that selective SGLT2 inhibition was required when it makes no claim of selectivity for dapagliflozin.
- Moreover, I do not read paragraph [0008] as saying that selective SGLT2 inhibition is essential. It gives the lack of selectivity of phlorizin as one of two reasons why phlorizin is unattractive as a drug. But the lack of selectivity is only said to be a potential problem because it could cause adverse effects. To the extent relevant, that was consistent with the evidence of Prof. Thorens and Prof. Bailey.
- In my judgment SGLT2 selectivity should not be treated as a requirement in itself for utility in treating etc. diabetes. The question to be addressed when considering plausibility of dapagliflozin as being useful to treat etc. diabetes (as well as an experimental tool to lower blood glucose in vivo) relates to efficacy, which is consistent with the position described in paragraphs 20-22 above.
- Accordingly, I conclude that what it means to "work" for the purpose of the second Fibrogen step in respect of claim 15 is treating etc. diabetes, in the sense of having sufficient efficacy to treat diabetes, and in respect of claim 2 is either (a) the same as in the case of claim 15 or (b) reducing blood or plasma glucose in vivo with sufficient efficacy to allow it to be used as an experimental tool.
The third Fibrogen step
- I now turn to consider the third Fibrogen step.
- Prof. Thorens and Prof. Bailey were in agreement that the mechanism proposed in the Patent, i.e. that SGLT2 inhibition would lead to a reduction of blood / plasma glucose in vivo and hence an effect on the diabetes disease state (see paragraphs [0002] and [0004] quoted in paragraph 213 above) was a plausible one, given the support provided by the phlorizin and Tanabe Seiyaku studies referred to in paragraphs [0004] and [0007].
- The question is whether the Patent discloses enough to make it plausible that dapagliflozin has activity as an SGLT2 inhibitor which is sufficient to have a useful effect on blood / plasma glucose levels in vivo and/or on the diabetes disease state.
- As can be seen from my discussion of the disclosure of the Patent above, on numerous occasions the Patent refers to dapagliflozin as an SGLT2 inhibitor (see e.g. the title, paragraphs [0019], [0051]-[0053], [0074] and [0102] and claim 15). The parties took very different views of whether that was a bare assertion or the statement of an experimental result. This was, AZ said, the central question in the case.
- AZ said that the Patent disclosed that the assay described in paragraph [0115] had been performed on dapagliflozin, and so the statements that dapagliflozin was an SGLT2 inhibitor would be understood as verbal statements of the result of that assay. It pointed out that the description of the assay in paragraph [0115] was in the past tense, e.g. "Evaluation of inhibition of SGLT2 activity...was performed essentially as described in Ryan et al.... Cells were then incubated with 10 mM [14C]AMG, and 10 mM inhibitor... For determination of EC50 values, 10 inhibitor concentrations were used over 2 log intervals in the appropriate response range, and triplicate plates were averaged across plates."
- However, as the Claimants pointed out, paragraph [0114] says (emphasis added): "SGLT2 inhibitor activity of the compounds of the invention may be determined by use of an assay system as set out below." Further, the description of the assay in paragraph [0115] does not say that dapagliflozin was assayed. Instead it says that what was used in the assay was "10 mM inhibitor". There is nothing to tell the reader what the inhibitor was.
- Further, I accept the Claimants' submission, supported by their cross-examination of Prof. Bailey, that the skilled person reading the Patent would understand that the assay could have been performed by the patentee on other compounds, either in the validation of the assay, in which case phlorizin might have been used as the inhibitor, or in assessing the SGLT2 inhibitory activity of other compounds. The fact that paragraph [0115] tells the reader that the assay has been performed on an inhibitor does not tell the reader that it has been performed on dapagliflozin.
- The reader might guess that had been done, but that is not the same as a disclosure that it has been. While Prof. Bailey and Prof. Potter said that the reader would understand that the assay had been performed on dapagliflozin, that was based on their assumption that a pharmaceutical company would not apply for a patent (let alone be granted one) if it did not have data to support the assertion that dapagliflozin was an SGLT2 inhibitor. That is a bootstraps argument, and in my judgment the assumption is not a permissible one to make (see paragraph 17 above). Further, both Dr Edwards and Prof. Potter said that the fact that 20g dapagliflozin had been made did not make any difference to the assessment of whether it had been assayed.
- Overall, I reject AZ's characterisation of the Patent's description of dapagliflozin as an SGLT2 inhibitor as a verbal statement of an experimental result. On the contrary, it is an assertion unsupported by any experimental results.
- As Warner-Lambert makes clear, a priori reasoning can provide an alternative basis for plausibility of an effect. However, the Patent contains no reasoning, based on structural similarities or otherwise, to support the suggestion that dapagliflozin is an SGLT2 inhibitor.
- AZ relied on the similarities between dapagliflozin and phlorizin, which it said would provide the skilled team with "additional reassurance" that dapagliflozin would be an SGLT2 inhibitor. The structure of phlorizin is shown below, together with that of the Tanabe Seiyaku compound T-1095a:
|
Phlorizin |
T-1095a |
|
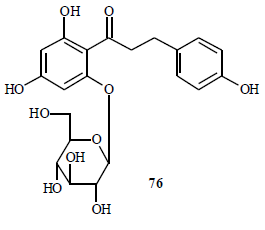
|
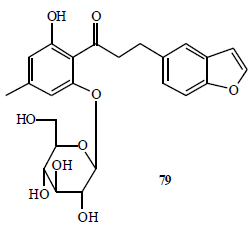
|
- A comparison of the structures of phlorizin and dapagliflozin shows the following: (i) both contain a glucose moiety in the b configuration; (ii) both contain two phenyl rings; (iii) in phlorizin the first phenyl ring is linked to the glucose by an oxygen whereas in dapagliflozin it is linked directly to the glucose moiety by a carbon-carbon bond, which the evidence showed will change the spatial relationship between the first phenyl ring and the glucose moiety and could affect the electronics of the phenyl ring; (iii) the first phenyl ring in phlorizin has two hydroxy groups meta to the oxygen link to the glucose while in dapagliflozin there is a chloro group para to the link to the glucose; (iv) in phlorizin the linker between the two phenyl rings is -CO-CH2-CH2- ortho to the oxygen link to the glucose (and the evidence established that the carbonyl in the linker would be likely to hydrogen bond with the hydroxy group ortho to it on the first phenyl ring) whereas in dapagliflozin the linker is -CH2- meta to the link to the glucose; (v) in phlorizin the second phenyl ring has an -OH group para to the linker to the first phenyl ring whereas in dapagliflozin that group is -OEt.
- It was quite clear from the evidence of Dr Edwards and Prof. Potter that, given the CGK that small structural differences can make large differences to activity, the number and nature of the differences between dapagliflozin and phlorizin were such that no prediction could be made, based on the activity of phlorizin, about the activity of dapagliflozin as an SGLT2 inhibitor. The most that could be said was that the removal of the O-glucoside bond might reduce the risk of hydrolysis of dapagliflozin compared to phlorizin, and that the glucose moiety in dapagliflozin might interact with SGLT2. However, I accept Dr Edwards' evidence that the presence of the glucose moiety, together with the two phenyl rings, does not provide the skilled team with the ability to make a reasonable prediction that dapagliflozin would be an SGLT2 inhibitor.
- Indeed, AZ accepted that the similarities (such as they are) with phlorizin would not on their own be sufficient to make plausible the assertion that dapagliflozin was an SGLT2 inhibitor. It relied on them only to "reinforce the confidence" that the skilled team would be given by "the positive assay result". However, in my judgment there is no "positive assay result" disclosed in the Patent for the reasons explained above.
- There was a debate between the parties about whether the skilled team presented with the Patent would obtain and study any other documents and if so which. In Akebia v Fibrogen at first instance ([2020] EWHC 866 (Pat) ("Fibrogen HC")) Arnold LJ held (at [218]) that "there is no principle of law that the skilled team are deemed to read all documents cited in a patent. It is a context- and fact-dependent question, and thus it depends firstly upon the wording of the specification and secondly on the evidence." On appeal, Birss LJ did not decide whether that was correct, though he clearly thought there was merit in the argument that in that case the skilled person would take into account, when considering sufficiency, documents expressly characterised as ones in which compounds which might be used in the invention could be found (see Fibrogen CA at [148]). I shall apply the approach in Fibrogen HC, which I regard as consistent with the decision of the Court of Appeal in Halliburton v Smith [2006] EWCA Civ 1715 at [64]-[69].
- Prof. Potter's view was that the skilled medicinal chemist reading the Patent would see the reference to WO 128 at paragraph [0011] and look it up. I accept that - in my judgment a skilled medicinal chemist presented with a statement that dapagliflozin is an SGLT2 inhibitor but no experimental data to support it, and a statement in paragraph [0011] that compounds of a Markush formula which covers dapagliflozin have been reported in WO 128 to be SGLT2 inhibitors, would want to obtain WO 128 to see whether it contained SAR information that could be used to assess the claim of SGLT2 inhibition for dapagliflozin. Of course, if the skilled medicinal chemist were to obtain WO 128, they would find that it too contains no experimental data, and therefore no SAR information which might make it plausible that dapagliflozin was an SGLT2 inhibitor, let alone indicate what its EC50 might be.
- Prof. Thorens and Prof. Potter said that the skilled team presented with the statements in paragraphs [0004] and [0007] of the Patent about the Tanabe Seiyaku 6 month rat study (or their equivalents in WO 128) would want to find out more about that work. However, no attempt was made to recreate the kind of search that would have been done at the priority date to locate that work. The skilled team which had WO 128 would, however, see that it contained a number of references to prior work on compounds which are said to be SGLT2 inhibitors. Those references include the papers by Tsujihara et al., Hongu et al. and Oku et al. referred to in paragraph 161 above; in my judgment the skilled team would regard those as potential sources for the Tanabe Seiyaku work referred to in paragraphs [0004] and [0007]. Further, Prof. Potter said that the skilled medicinal chemist would want to look at materials cited in WO 128, including those papers, and would be interested in any SAR information they contained. Therefore, in my judgment the skilled team which had WO 128 (which for the reasons explained in the previous paragraph, the skilled team reading the Patent would have done) would also look at the four Tanabe Seiyaku papers which it cites.
- Even a brief consideration of those papers would reveal that the SAR work conducted by Tanabe Seiyaku indicated strict structural requirements for activity of phlorizin analogues. The position can be summarised by setting out the abstract of the first Hongu et al. paper (which follows on from the Tsujihara et al. paper which had shown a benefit of removing the 4'-hydroxy group from the first phenyl ring, referred to as the B ring by Tanabe Seiyaku):
"A novel series of 4'-dehydroxyphlorizin derivatives was synthesised and the effects of these compounds on urinary glucose excretion were evaluated in rats. There was a strict structural requirement for activity. Introduction of a small substituent or a flat ring at the 3- and/or the 4-position on the A ring was permissible, but any change at the bridge part between the A and B rings or in the sugar moiety resulted in complete lack of activity. The 6'-OH group on the B ring was also necessary, and even small structural modifications of the 6'-OH group reduced the activity considerably. Among the compounds synthesised, the 5-benzofuryl derivative 25 [T-1095a] was the most potent and was selected as a new lead for further structure-activity relationship investigations."
- This emphasises the importance, in phlorizin analogues, of retaining the structure of the linker between the two phenyl rings, and the hydroxy group on the first phenyl ring that has the ability to hydrogen bond to the carbonyl in the linker. Both those features are absent in dapagliflozin, and so the skilled team considering the Tanabe Seiyaku papers would find nothing to suggest that dapagliflozin would be an SGLT2 inhibitor. Indeed, in my judgment the message that they convey, namely that structural features of phlorizin analogues (which are absent from dapagliflozin) are important for activity, would give the skilled team reason to doubt whether dapagliflozin would be an SGLT2 inhibitor.
- As neither WO 128, nor the Tanabe Seiyaku papers which it cites, contain any information to support the suggestion that dapagliflozin is an SGLT2 inhibitor, it is not necessary to consider whether it would be permissible to rely on information in these cross-referenced documents to establish or support a case of plausibility applying the approach in Warner-Lambert.
- AZ repeatedly asserted that the teaching of the Patent was that "positive results" had been obtained in the assay of dapagliflozin. However, even if one were to accept (which I do not) that the teaching of the Patent was that dapagliflozin had been assayed, that begs the question as to what the "positive results" were. Even if the skilled person were to understand the Patent as disclosing that dapagliflozin had been assayed and determined to be an SGLT2 inhibitor, it does not disclose the EC50 that had been obtained (or even how it compared with that of phlorizin).
- Nor, on the evidence, did the description of the assay mean that any positive result must have been a EC50 in a range which would confer utility. Dr Edwards said, and Prof. Bailey accepted, that the potency could have been in the millimolar range. AZ submitted that the assay could have been one with a 10 mM EC50 cut-off, and that a compound which passed such an assay would have been a "hit". I do not think there was anything in the evidence to suggest that the skilled team would have understood the assay to have such a cut-off, but in any event, on the evidence a "hit" in itself is not a compound with practical utility (see also Apixaban HC at [232]).
- AZ submitted that the statements in the Patent that dapagliflozin was an SGLT2 inhibitor useful for the treatment of diabetes must be read as teaching that the EC50 for dapagliflozin was such that it would be useful to treat diabetes. I agree with the Claimants' characterisation of this as another bootstraps argument. On the contrary, the Patent makes bare assertions that dapagliflozin is an SGLT2 inhibitor and useful to treat diabetes.
- The following passages of cross-examination of Prof. Bailey illustrate the position:
Q. Even if this assay was workable, there is no indication of threshold for the assay in this text?
A. By "threshold", could I just ask ----
Q. Yes, the skilled reader has no idea as to what counts as signifying activity as an EC50?
A. In terms of what is written here, you are correct.
Q. So, they have no idea what efficacy of the SGLT2 inhibitor the compound needs to be?
A. You would not be able to derive potency from the information that is given here; correct.
Q. As a result, you have no idea what utility any SGLT inhibitor might have?
A. Yes, you are correct.
Q. It could, for instance, be the millimolar potency which you accepted earlier would not be good enough to take forward?
A. It could be one you would, yes, not choose to carry forward.
Q. You just do not know what has been tested in the assay in the patent, Professor.
A. You are correct that I do not know what was tested; yes.
Q. Not only is there no scientific rationale to support any of the bare statements in the patent about SGLT2 inhibition, there is even less to tell you that dapag is effective as an SGLT2 inhibitor at a level which provides any utility whether to treat diabetes or otherwise.
A. We do not know the level, yes, or the potency.
Q. Yes. We do not know how effective it needs to be.
A. Yes, we probably do not at this stage.
- Overall, the evidence was that not every compound which produced a measurable EC50 in an in vitro SGLT2 inhibition assay would have been regarded as plausibly having a useful effect on blood / plasma glucose in vivo or the diabetes disease state. Indeed, while my conclusion does not depend on this, the skilled team which had the Tanabe Seiyaku papers cited in WO 128 would, as Prof. Potter accepted, note that Tsujihara et al. presents results showing that some compounds which had SGLT2 activity in vitro were indistinguishable from the control in vivo and so were categorised as inactive.
- The skilled team which had the Tanabe Seiyaku papers cited in WO 128 would also note that Oku et al. provides the IC50s for phlorizin (160 nM against both SGLT1 and SGLT2) and for T-1095a (20 nM against SGLT1 and 5 nM against SGLT2). It also presents the results of a study of T-1095a in diabetic rats, though it does not appear to be the study referred to in paragraphs [0004] and [0007] of the Patent (the source of that study was not clear from the materials in the case). There is nothing in the Tanabe Seiyaku papers to displace the skilled team's CGK about potency requirements for an SGLT2 inhibitor which could be useful to treat diabetes. They provide the IC50 for phlorizin as a benchmark for someone seeking an experimental tool to lower blood / plasma glucose. However, as I have said, the Patent provides no information on the EC50/IC50 of dapagliflozin.
- The absence of information about the EC50 of dapagliflozin is significant. The skilled team cannot reasonably predict any useful effect on blood / plasma glucose or on the diabetes disease state from merely being told that dapagliflozin is an SGLT2 inhibitor.
- For the reasons I have given above, in my judgment the Patent does not disclose enough to make it plausible that dapagliflozin will have an in vivo effect on blood / plasma glucose (such that it could be used as an experimental tool) or will treat etc. diabetes.
- The Claimants also took a point that claim 15 covered treating pre-diabetes. Their suggestion seemed to be that this gave rise to a problem for AZ because pre-diabetes was not a recognised disease regarded as suitable for treatment. I did not really understand this point. I agree with AZ that if it had been plausible that dapagliflozin would treat diabetes, there is no reason to think it would not also treat pre-diabetes.
- I have considered if I should decide whether my findings of fact mean that the Patent would pass a "legitimate reason for doubt" test. In the end I have concluded that I should not do so. That would require me to examine the nature of such a test - for example, whether the "legitimate reason for doubt" test of T 116/18 is the same as the "ab initio implausibility" test that preceded G 2/21 - and any attempt to do so would involve second guessing what a higher court might decide about the appropriate test. Further, as I have said, AZ did not identify any further findings of primary fact that it said needed to be made for the application of a "legitimate reason for doubt" test, nor have I been able to identify any findings of fact (over and above those I have already made) that I need to make, and can make on the evidence before me, to enable a higher court to apply whatever test it decides is appropriate following G 2/21.
Arbitrary selection
- AZ observed that the case of arbitrary selection (or, to put it another way, lack of technical contribution over WO 128) needed to be considered against the backdrop of the Claimants' (a) acceptance that WO 128 did not contain an individualised disclosure of dapagliflozin, and (b) abandonment of the case of classical obviousness (i.e. that it would have been obvious, given WO 128, for the skilled person to have alighted upon dapagliflozin as an SGLT2 inhibitor).
- AZ submitted that the case law of the EPO and the UK established the principle that "if the alleged selection is not classically obvious - it is inventive - then that is the end of any suggestion of arbitrariness". It submitted that "there is no such thing as an arbitrary invention".
- I do not agree that the case law establishes any such principle. On the contrary, the case law emphasises that a patent may be invalid in circumstances where the claimed invention is a mere arbitrary selection from the prior art (or to put it another way, where the patent does not plausibly and/or in fact make a technical contribution to the art), regardless of whether there is anything to point the skilled person towards the claimed invention. Indeed whereas AZ relied, in response to the classical obviousness case, on the absence of any pointer in the prior art to dapagliflozin, it accepted that no such pointer was needed in the case of an arbitrary selection. That in itself shows that the failure of the Claimants to establish classical obviousness does not mean "the end of any suggestion of arbitrariness".
- Next, it is important to note AZ's position as set out in its opening skeleton argument:
"AZ need not establish that dapagliflozin is a more effective treatment than any specific compound encompassed within the teaching of WO 128 (either as a matter of plausibility or of fact). That is particularly so because WO 128 does not plausibly establish that each alternative compound encompassed within its disclosure accesses the technical contributions on which AZ relies (namely that each of them is (i) an SGLT2 inhibitor, and/or (ii) useful in the treatment of diabetes). AZ therefore does not need to rely on a technical contribution that dapagliflozin is more effective than any particular compound identified in WO 128."
- In other words, AZ conducted the trial on the basis that it was not necessary for dapagliflozin to have greater efficacy than (or be in any other way superior to) any of the other compounds encompassed by the disclosure of WO 128, including the compounds of the Examples. That was consistent with its decision, noted in paragraph 222 above, not to rely on its pleaded technical contributions (iii) and (iv) which involved comparisons of the properties of dapagliflozin with those of the compounds of Example 12 (the -OMe analogue of dapagliflozin) and Example 10, and with its decision not to rely at trial on any document comparing or otherwise indicating those properties.
- The technical contributions relied upon by AZ at trial were, as explained earlier, that dapagliflozin was an SGLT2 inhibitor which reduced blood / plasma glucose in vivo (and so could be used as an experimental tool) and/or was useful in the treatment etc. of diabetes. But AZ did not advance a case that dapagliflozin was, in those regards, in fact any different in its properties to any of the other compounds of WO 128 (and in particular to the compound of Example 12).
- For the reasons explained when considering the law above, in my judgment that means that the Patent does not make a technical contribution over WO 128. There is nothing in the Patent nor in the evidence at trial to indicate that dapagliflozin is anything other than an arbitrary selection from the genus of compounds disclosed by WO 128.
- The argument advanced by AZ was that the Patent made it plausible that dapagliflozin was an SGLT2 inhibitor which reduced blood / plasma glucose in vivo and was useful to treat etc. diabetes, whereas that was not made plausible by WO 128. That, AZ submitted, taught the skilled person something new and so was a technical contribution.
- In my judgment there is some sleight of hand going on here. A technical contribution needs to be both present in fact and made plausible by the specification (hence Birss J's questions in Takeda v Roche included "is it plausible?", "is it true?" and "is it a technical advance?" and Jacob LJ in Dr Reddy's said that one should ask whether the patentee had made a technical advance and provided sufficient justification for it to be credible). I do not see how making something plausible can in itself be a technical contribution. Indeed AZ did not plead a technical contribution consisting of making something plausible - it pleaded (correctly, in my judgment) actual properties of dapagliflozin as being technical contributions.
- Even if this argument were a permissible one, in my judgment it does not work in this case. The parties were agreed that, in order for the arbitrary selection case to add anything to the plausibility case, it is necessary to assume that the Patent does make it plausible that dapagliflozin is an SGLT2 inhibitor which reduces blood / plasma glucose in vivo and/or is useful to treat etc. diabetes. That requires me to approach matters on the basis that the Patent would be understood as disclosing that dapagliflozin had been assayed and had generated an EC50 which meant that it would plausibly reduce blood / plasma glucose in vivo and/or be useful to treat etc. diabetes.
- If that is so, then in my judgment WO 128 must also be understood as disclosing that compounds which it discloses had been assayed and had generated EC50s which meant that they would plausibly reduce blood / plasma glucose in vivo and/or be useful to treat etc. diabetes. Prof. Potter (whose view was that the skilled medicinal chemist reading the Patent would assume that dapagliflozin had been tested) said this:
"While the Medicinal Chemist would not be able to identify which of the compounds encompassed by WO 128 were tested in the assay, they would not doubt that the assay had in fact been used in relation to some of them. While no biological data are provided, it would be a reasonable assumption that at least the Examples - for each of which characterisation data are provided, indicating that they were synthesised - would have been tested for activity using this assay."
- Therefore, on this approach the skilled team would understand that the compound of Example 12 of WO 128 (amongst others) had been tested and produced an EC50 which meant that it would plausibly reduce blood / plasma glucose in vivo and/or be useful to treat etc. diabetes.
- Prof. Potter also said that:
"I do not believe that the Medicinal Chemist would have any reason to doubt the assertion in WO 128 that the class of compounds of Structure IB do inhibit SGLT2, albeit they may not expect all of the very large number of compounds captured by Structure IB to have this effect."
- My understanding of Prof. Potter's evidence was that the skilled medicinal chemist would regard the statement in WO 128 that the compounds of formula IB are SGLT2 inhibitors and useful in the treatment etc. of diabetes as being a prediction based on assay results of the compounds of the Examples, and would regard the prediction as being a reasonable one, albeit recognising that it might not be true in respect of each and every one of the compounds covered by formula IB.
- There was no suggestion that any particular compounds covered by formula IB would be regarded as likely exceptions to the prediction - in particular there was no suggestion that the skilled medicinal chemist would regard a compound with R1 = Cl and/or R4 = OEt as being a likely exception. On the contrary, Prof. Potter's evidence was that the skilled medicinal chemist would have no expectation that a compound with an alternative alkoxy group would have better, the same or worse activity data than one with a methoxy group (as in Example 12).
- Therefore, on the hypothesis on which I am considering matters, WO 128 contains what the skilled team would regard as a plausible prediction, based on assay data for the compounds of the Examples, that compounds of formula IB (including dapagliflozin with its close relationship to Example 12) will be SGLT2 inhibitors and that they will reduce blood / plasma glucose in vivo and/or be useful to treat etc. diabetes.
- All that the Patent does (on that hypothesis) is to verify that WO 128's prediction of SGLT2 inhibitory activity and utility is correct in the case of dapagliflozin. It does not show that dapagliflozin has properties that are in any way different from those predicted by WO 128. On that hypothesis, while the Patent may make it more plausible that dapagliflozin will reduce blood / plasma glucose in vivo and/or be useful to treat etc. diabetes, it does not make that plausible for the first time. So I reject AZ's argument which I have recorded in paragraph 270 above.
Conclusion
- For the reasons explained above, I conclude that the Patent was invalid and, accordingly, the SPCs are invalid.
- I recognise that, as I said at the outset, dapagliflozin has proved to be successful but, to echo what Meade J said in Apixaban HC at [21], while no judge wants to revoke a patent for a breakthrough, later findings about dapagliflozin do not enter the picture and my task has been to assess the validity of the Patent based on its disclosure.
- It is not for me to speculate about why the Patent was drafted in the way it was, and in particular why it contained no data about the performance of dapagliflozin in any assay. If it had done so, the outcome of these proceedings could well have been different; indeed they may never have taken place at all.