Introduction
1. This is a patent action about DNA sequencing technology. The patentee (Illumina) holds patents which derive from work by Solexa, a spin out company from Cambridge University which Illumina bought in 2007. The defendants (MGI) are all companies in the Beijing Genomics Institute group. MGI seeks to sell DNA sequencing systems in the UK. Illumina contends that these systems infringe various of its patents. In general, MGI denies infringement of any valid claim and contends the patents are invalid. Following a launch last year, MGI gave undertakings limiting UK sales until this trial.
2. It is convenient to take three Illumina patents together. They are EP (UK) No. 1 530  578,
578, EP (UK) No. 3 002 289 and EP (UK) No. 3 587 433. These three patents are divisionals. The first two are entitled “Modified Nucleotides for Polynucleotide Sequencing” and 433 is entitled “Modified Nucleotides”. They based on an application filed on 22nd August 2003. Although the earliest claimed priority is a US filing on 23rd August 2002, in this case Illumina relied on the second priority document with a priority date of 23rd December 2002. The three patents were granted on 13th March 2013, 22nd February 2018 and 22nd April 2020 respectively. The
EP (UK) No. 3 002 289 and EP (UK) No. 3 587 433. These three patents are divisionals. The first two are entitled “Modified Nucleotides for Polynucleotide Sequencing” and 433 is entitled “Modified Nucleotides”. They based on an application filed on 22nd August 2003. Although the earliest claimed priority is a US filing on 23rd August 2002, in this case Illumina relied on the second priority document with a priority date of 23rd December 2002. The three patents were granted on 13th March 2013, 22nd February 2018 and 22nd April 2020 respectively. The  578
578 patent was opposed at the EPO but those proceedings ended with the patent upheld as granted. Opposition proceedings relating to the 289 continue and the opposition period for 433 has not yet ended. These patents all relate to an azidomethyl group as a reversible chain terminator in sequencing by synthesis.
patent was opposed at the EPO but those proceedings ended with the patent upheld as granted. Opposition proceedings relating to the 289 continue and the opposition period for 433 has not yet ended. These patents all relate to an azidomethyl group as a reversible chain terminator in sequencing by synthesis.
3. EP (UK) 1 828 412 is entitled “Improved Method of Nucleotide Detection”. It was filed on 13th December 2005 with its earliest claimed priority being a UK filing on 13th December 2004. It was granted on 28th November 2012. Opposition proceedings were commenced but have now been finally concluded. The patent was upheld in an amended form. The patent relates to the use of ascorbic acid (or a salt thereof) as a component in the fluorescent imaging buffer. Ascorbic acid is an anti-oxidant.
4. EP (UK) 2 021 415 is entitled “Dye Compounds and the use of their Labelled Conjugates”. It was filed on 16th May 2007 claiming priority from a US UK filing on 18th May 2006. It was granted on 15th March 2017. There was no EPO opposition. As proposed to be amended the patent relates to a conjugate molecule consisting of a nucleotide, a particular cleavable linker and a particular fluorescent dye compound.
The modified nucleotide patents - issues
5. In terms of validity, MGI pleaded that the modified nucleotide patents are obvious over four pieces of prior art:
i) International patent application WO 91/06678 (Tsien) filed by SRI International and published on 16th May 1991;
ii) International patent application WO 02/29003 (Ju) filed by a group at Columbia University and published on 11th April 2002;
iii) A paper entitled “1-Alkythioalkylation of Nucleoside Hydroxyl Functions and Its Synthetic Applications: A New Versatile Method in Nucleoside Chemistry”, Zavgorodny et al., Tetrahedron Letters (1991) Vol. 32, No. 51, pp 7593-7596; and
iv) A paper entitled “S,X-acetals in nucleoside chemistry. III1. Synthesis of 2’- and 3’-O-azidomethyl derivatives of ribonucleosides”, Zavgordony et al., Nucleosides, Nucleotides and Nucleic Acids (2000) Vol. 19, Issue 10-12, pp1977-1991.
6. The written evidence covered all four citations, however shortly before trial MGI abandoned its case on Tsien and on Ju. By closing it became clear that there was no need to dwell on Zavgorodny 2000. MGI’s case can be made over Zavgorodny 1991 and if that does not succeed then the case over Zavgorodny 2000 would not succeed either.
7. There is also an issue of priority. If the modified nucleotide patents are not entitled to the December 2002 priority date then a further citation is prior art and is relied on for obviousness: US patent application no. 2003/0104437 A1, published on 5th June 2003. This was a Solexa application and has been called “Barnes”, after the first named inventor.
8. Another validity question is whether particular claims of  578
578 and 433 are obvious for lack of technical contribution and/or insufficient. The issue is the same for both claims. Illumina advances an amendment which (it is not disputed) would cure that invalidity but does not agree those unamended claims are invalid and so the point falls to be decided. The relevant claims are 12 (as granted) of
and 433 are obvious for lack of technical contribution and/or insufficient. The issue is the same for both claims. Illumina advances an amendment which (it is not disputed) would cure that invalidity but does not agree those unamended claims are invalid and so the point falls to be decided. The relevant claims are 12 (as granted) of  578
578 (now claim 7 of claim set A) and claim 6 of 433 (as granted and in claim set C).
(now claim 7 of claim set A) and claim 6 of 433 (as granted and in claim set C).
9. There were two added matter objections to the claim amendments. The one which remains live relates to claim 9 of 289 (claim set B). The one which was dropped was a challenge to claim 1 of  578
578 (claim set A). MGI dropped it after Illumina changed the amendments it was seeking to
(claim set A). MGI dropped it after Illumina changed the amendments it was seeking to  578
578 by deleting granted claim 8. Both added matter issues are referred to at Illumina MNP Issue 6 but as explained only one is live.
by deleting granted claim 8. Both added matter issues are referred to at Illumina MNP Issue 6 but as explained only one is live.
10. Finally there is an insufficiency squeeze in relation to a number of the relevant claims of the modified nucleotide patents such that, if they are not obvious, they are insufficient, in part having regard to the recent Supreme Court decision in Regeneron v Kymab [2020] UKSC 27.
11. In terms of infringement, MGI has various systems alleged to infringe. One system is called StandardMPS and the other is called CoolMPS. Both use the azidomethyl group on the deoxyribose as a reversible chain terminator. In StandardMPS the four nucleobases carry a different fluorescent dye molecule covalently linked to the base via a linker. In CoolMPS the nucleobase is not covalently linked to a dye, rather detection uses four different antibody molecules, each linked to a different dye and each of which binds to a different nucleobase and the azidomethyl group. There are various detailed infringement issues, including allegations based on the doctrine of equivalents.
12. MGI also has two further azidomethyl based systems. They are the “two colour variant” and something called DNBSEQ E. In the two colour variant instead of four different dyes linked to the four nucleotides as in Standard MPS, only two dyes are used and detection occurs in two colours. Just as two binary bits can encode four numbers, so two dyes can distinguish four nucleotides by putting one dye on one nucleotide, the other dye on another nucleotide, both dyes on a third nucleotide, and no dye on the fourth nucleotide.
13. In the DNBSEQ E variant there are no fluorescent dyes at all. The nucleotides are linked to two types of non-fluorescent label. This method uses the same kind of encoding scheme as the two colour variant to distinguish four nucleotides.
14. All four of StandardMPS, CoolMPS, the two colour variant and the DNBSEQ E are alleged to infringe some claims of the modified nucleotide patents. Some of those points are admitted and others are not. The very useful lists of issues provided in closing naturally only list the points which are in dispute, but to get a full picture one needs to see the admitted aspects as well. A useful summary of the whole position was provided by Illumina. I have adjusted the claim numbers in it. In summary the position is:
StandardMPS, the two colour variant and the DNBSEQ E variant
i) Claims 1, 7, 12, 20 and 24 of the  578
578 patent (claim set A) are alleged to be infringed by all three systems. MGI does not admit infringement of claim 20 (claim set A) by the two colour or DNBSEQ E variants. The other allegations are admitted.
patent (claim set A) are alleged to be infringed by all three systems. MGI does not admit infringement of claim 20 (claim set A) by the two colour or DNBSEQ E variants. The other allegations are admitted.
ii) Claims 1, 4, 5 and 6 of the 289 patent (claim set B) are alleged to be infringed by all three systems. MGI does not admit infringement of claim 4 by the two colour variant kit or the DNBSEQ E variant kit. The other allegations are admitted.
iii) Claims 1 and 6 of the 433 patent (claim set C) are admitted to be infringed by all three systems.
Cool MPS
iv) It is alleged that the CoolMPS system infringes claims 1, 7, 12, 20 and 24 of the  578
578 patent (claim set A). Infringement of claims 1 and 24 (dependent on claim 1) (claim set A) is admitted. Infringement of claims 7, 12 and 20 of the
patent (claim set A). Infringement of claims 1 and 24 (dependent on claim 1) (claim set A) is admitted. Infringement of claims 7, 12 and 20 of the  578
578 patent (claim set A) is in issue. The points on normal construction relate to cleavable linker, incorporation and base/blocking group attachment. The points on equivalents relate to cleavable linker and incorporation.
patent (claim set A) is in issue. The points on normal construction relate to cleavable linker, incorporation and base/blocking group attachment. The points on equivalents relate to cleavable linker and incorporation.
v) It is alleged that the CoolMPS system infringes claims 1, 4, 5 and 6 of the 289 patent (claim set B). Infringement of claims 1 and 5 of the 289 patent is admitted. Infringement of claims 4 and 6 of the 289 patent is in issue. The point relates to cleavable linker on both a normal construction and doctrine of equivalents.
vi) It is admitted that the CoolMPS system infringes claims 1 and 6 of the 433 patent (claim set C).
15. At the end of its list of issues MGI raised a point (MGI MNP Issue 12) about declarations under s71 of the 1977 Act. The point was not argued in any detail and Illumina objected to dealing with it in this way. I am not in a position to decide anything about it in this judgment. If the point is still live then the way forward must be for MGI to make an application for whatever order they are asking the court to make.
The fluorescence issues - the 412 and 415 patents
16. The 412 patent is alleged to be obvious over US Patent No. 6,544,797 (Buechler) published on 8th April 2003. There is also an issue about added matter.
17. Illumina proposed a conditional amendment to claim 1. The amendment is advanced as a way to cure the added matter problem if, which Illumina denies, the added matter point succeeds. MGI does not contend the amendment per se is not allowable but argues that it does not cure the added matter. It makes no difference to infringement.
18. On infringement of the 412 patent, MGI admits that Standard MPS infringes claims 1 and 15. As I understand it that admission includes the two colour variant as well. Illumina does not assert infringement of the 412 patent by the DNBSEQ E variant. Illumina does assert that CoolMPS infringes claim 1 of the 412 patent both on a normal construction and by the doctrine of equivalents, and these arguments are denied by MGI.
19. For the 415 patent there is an unopposed application to amend down to claim 3 as granted. Infringement by StandardMPS of claim 1 as proposed to be amended was admitted. Illumina did not allege infringement by any of the other three systems.
20. The issues on the 415 patent all relate to invalidity. The prior art relied on is US Patent No. 4,900,686 (Arnost) published on 13th February 1990 and PCT Application No. WO 2004/018493 (Milton) published on 4th March 2004. The former (Arnost) relates to fluorescent dye compounds and the latter (Milton) relates to linkers. MGI advances a collocation argument based on these two documents. There is also an Agrevo / lack of technical contribution obviousness argument. There had been a point on insufficiency but it was dropped.
The trial
21. Given the pandemic, the trial was conducted as a hybrid trial with the core legal teams in the physical court room along with me, and the rest of the legal teams working remotely. All bar one of the witnesses gave their evidence remotely. For the two witnesses giving evidence from Germany (Prof Dr. Marx and Prof Johnsson), suitable arrangements were made with the Amtsgericht Freiburg im Breisgau pursuant to Art. 17 of the Council Regulation (EC) No. 1206/2001 so that the witnesses could give their evidence by video link from Germany. For Prof Winssinger in Switzerland, arrangements were made with the Swiss Federal Dept of Justice and Police in the relevant Canton (Vaud) under Art. 17 of the Hague Convention (1970). I am grateful both to the Freiburg Court and the Swiss FDJP for their assistance in this matter. The defendants’ legal teams left it far too late to make these arrangements and it was only with the assistance and cooperation of those authorities (and the efficiency of the Masters of the Queen’s Bench Division) that the arrangements were made in time.
22. After the trial Illumina sent me an unsolicited note concerning Regeneron v Kymab. To forestall a proliferation of notes, I directed a short further hearing which took place (remotely) on 9th December to hear both sides on these issues.
The witnesses
23. Illumina called Professor Peter Leadlay as an expert to give evidence in relation to the modified nucleotide patents. Prof Leadlay is the Herchel Smith Professor of Biochemistry Emeritus at Cambridge, Fellow of the Royal Society and Fellow of the Royal Society of Chemistry. After studying chemistry at Oxford, Prof Leadlay held various academic positions at the ETH Zürich and at Oxford before moving to Cambridge in 1977 where he became Professor of Molecular Enzymology in 1998 and took the Herchel Smith Chair in 2006. Between 1993 and his retirement in 2018, Prof Leadlay directed the DNA Sequencing Facility in the Biochemistry Department at Cambridge.
24. MGI acknowledged that Prof Leadlay is a distinguished scientist and was good at explaining technical concepts (he was), but submitted that he was in a very unfortunate position of being asked to give evidence in relation to a field which was not his own and that as a result his evidence was of limited value to the court. I reject that submission. Prof Leadlay was the director of a major DNA sequencing laboratory at all material times. The fact he was not doing the day to day work himself does not disqualify him from speaking about it. As Prof Leadlay readily accepted, he was not trying to devise new sequencing machines. That does not disqualify him from giving evidence. Prof Leadlay’s experience and knowledge amply qualified him to assist the court on the issues relevant to the modified nucleotide patents. The fact the professor had not read the papers such as Metzker and Canard which MGI wanted to say were common general knowledge did not demonstrate he was not in the relevant field. For one thing that assumes the truth of a heavily disputed proposition MGI seeks to prove. It may amount to nothing more than a consequence of the fact he was not trying to develop new methods himself at the relevant date. It may serve as evidence contrary to MGI’s case. In any case the submission is another instance of the frequent fallacy in patent cases that the only experts qualified to comment have to have been working on the very problem the patent sets out to solve at the relevant time. That is wrong. The expert’s particular area of interest and work may well be a factor to take into account, depending on the circumstances, but it rarely justifies a submission of the kind advanced by MGI here.
25. MGI also submitted that Prof Leadlay’s attempt to “recreate” (as MGI put it) the common general knowledge was flawed, that he overreached himself and speculated to fill in gaps. I do not accept this characterisation of the witness at all. A particularly unfair submission is a criticism about evidence the professor gave in cross-examination about a conference in 1994. Never mind the fact that given a 2002 priority date nothing useful was likely to be gained from considering who may or may not have attended a single conference in 1994. The criticism is that Prof Leadlay “changed his tune” about who would have attended the conference when it was pointed out to him that the Metzker and Canard groups had presented at the conference. However Prof Leadlay did no such thing. I remember the oral evidence on this but I have taken the trouble to carefully re-read the whole of the relevant transcript. The professor’s evidence was consistent throughout. The fact he or his laboratory manager John Lester might have gone to it (but did not) is not inconsistent with his view that the skilled person, as the professor defined that person, would not have.
26. Another criticism is said to be the professor’s suggestion that the earliest Metzker and Canard work was not promising “even in 1994”, whereas he said the patent offered a breakthrough “even though” as MGI asserts “the data in each are comparable”. I will deal with the technical issues in context, but as a criticism of the witness this is also hopeless. The answers the professor gave to the question about 1994 were cogent and not inconsistent with his views about the patent(s) in suit.
27. The only other criticism of Prof Leadlay I will mention is that his evidence was said to be coloured by his own experience of thinking that Solexa were the first people to use reversible chain terminators in general. This is just another way of making the same point that Prof Leadlay had not read papers such as Metzker and Canard. It is not a reason to apply a general discount to his evidence. MGI also submit that the professor’s evidence was itself tainted with hindsight. It is certainly true that in principle hindsight can infect arguments advanced in favour of an inventive step, as well as arguments in favour of obviousness but if I find that has taken place I will deal with it in context.
28. None of MGI’s submissions about Prof Leadlay’s evidence lead me to think I should generally discount his evidence at all. On the contrary Prof Leadlay was a good witness, using his skill and knowledge to help the court understand the technical issues and decide this case. There are points of detail relating to particular pieces of evidence given by all the witnesses in this case, including Prof Leadlay. If they need to be addressed, they are best dealt with in context.
29. MGI called two expert witnesses in relation to the modified nucleotide patents. The first was Professor Dr. Andreas Marx, who is currently - and has been since 2004 - Professor of Organic Chemistry/Cellular Chemistry at the University of Konstanz in Germany. After studies in Chemistry at the Ruhr-Universität Bochum, Prof Marx obtained his D. Phil. at the University of Basel in organic/biological chemistry studying DNA polymerases and modified nucleotides. After a period in Japan at the Nagoya University, Prof Marx was a Group leader at the Kekulé-Institute of Organic Chemistry at the University of Bonn until 2004, where he completed the requisite qualification to become a professor in Germany specialising in organic chemistry and biochemistry.
30. Prof Marx was an excellent witness, clearly aiming to help the court and to explain his sincerely held opinions. I am grateful to him for his evidence.
31. The second of MGI’s witnesses in relation to the modified nucleotide patents was Professor Nicolas Winssinger, who is currently a professor in the Department of Organic Chemistry at the University of Geneva. Prof Winssinger studied science at Tufts University before doing doctoral and post-doctoral research at the Scripps Research Institute in San Diego, California. From 2002 - 2005, Prof Winssinger was an associate professor and director of the organic and bioorganic laboratory at the Institute of Science and Supramolecular Engineering at the Louis Pasteur University. Thereafter, Prof Winssinger was a full professor within the same institution, but at the University of Strasbourg, before he moved to the University of Geneva in 2012.
32. Although Illumina did not criticise Prof Winssinger for it, I was not impressed with the professor’s testimony in cross-examination. An issue relevant to obviousness was about the perceptions of the skilled person of the utility of Staudinger chemistry for reducing azides. Counsel put to Prof Winssinger that Staudinger was thought to be slow at the relevant temperatures and Prof Winssinger said he strongly disagreed. Counsel then put to the professor a scientific paper of his published in 2003 (Debaene and Winssinger) which involved the use of azides in the synthesis of peptide nucleic acids to mask the N terminus and used Staudinger chemistry for deprotection. The questions made the simple point that on the face of the paper, it seemed that in 2003 Prof Winssinger had regarded the Staudinger reduction as attractive due to its mildness, but as having impractically long reaction times. The professor did not accept that that was the right way to understand the paper. I am not concerned about whether the professor’s explanation of the context of the work in the paper in fact shows that the issue about timing it mentions is relevant in the present case or not. What troubled me was the blithe way Professor Winssinger treated something he had previously written (or at least was in his name). Chasing through the chemistry led to tests carried out on two azaylide compounds 10 and 11. They were part of the testing to fix the timing issue (or I think actually to resolve a knock on effect of the step taken to fix the timing issue, but it does not matter). The paper records (p4447 lower LH side) that he and his co-worker were “pleased to observe that compound 10 was completely consumed after 1 hr” but then stated that “it was interesting to note” that compound 11 (tributyl azaylide) “did not react under these conditions”. In other words, on the face of it, the fix was not so simple. One of the two compounds worked but “interestingly” (in his own words at the time) the other did not. In his answer (at T9/1044 lines 11-25) the professor did not face up to what had been written but instead sought to suggest it was not interesting at all but rather was just the result of the well established, text book, rules of organic chemistry. This was not the first argumentative answer from the professor but is the clearest example and shows a lack of objectivity on his part. He was arguing the case. He was not there seeking to give candid and objective evidence. To reject the entirety of his evidence would be a disproportionate response but I am doubtful I can place much weight on opinions expressed by Prof Winssinger which are not backed up by other evidence such as contemporaneous documents.
33. In relation to EP 412 and EP 415, Illumina called Professor Marc Greenberg. Since 2016 he has been the Vernon K Krieble Professor of Chemistry at the Johns Hopkins University. Prof Greenberg studied chemistry at New York University, before doing a PhD in chemistry at Yale. Prof Greenberg then did post-doctoral research as the American Cancer Society Postdoctoral Fellow at CalTech. In 1998, he moved to Colorado State University, where he became a professor in the Department of Chemistry in 1999. In 2002, Prof Greenberg moved to Johns Hopkins.
34. MGI called Professor Johnsson in relation to EP 412 and 415. Prof Johnsson is currently Director at the Max Planck Institute for Medical Research, Department of Chemical Biology in Heidelberg, a position he has held since 2017. Prof Johnsson studied chemistry at the ETH Zürich and subsequently undertook post-doctoral research in the USA (Berkeley) and Germany (Ruhr-Universität Bochum). From 1999 to 2017 Prof Johnsson held a number of academic positions at the Institute of Chemical Sciences and Engineering at the Swiss Federal Institute of Technology in Lausanne before he moved to the Max Planck Institute.
35. Both Prof Greenberg and Prof Johnsson were good witnesses, giving their sincerely held opinions and I am grateful to them both.
36. The parties also called three more professors as fact witnesses. Illumina called Professor John Mattick AO and Professor Michael Lovett. They were called to assist Illumina’s case that sequencing by synthesis using reversible chain terminators (RCT) was not common general knowledge, particularly at the earlier priority dates in this case (2002 and 2004). MGI called Prof George Church, largely to address the same point.
37. Prof Mattick is the SHARP Professor of RNA Biology at the University of New South Wales, Sydney. Prof Lovett is the Chair in Systems Biology at the National Heart and Lung Institute at Imperial College. Prof Church is Professor of Genetics at Harvard Medical School. MGI chose not to cross-examine Profs Mattick and Lovett. Prof Church was cross-examined. He was a good witness and Illumina did not criticise his evidence. I am grateful to all three of these professors for their evidence.
The witnesses not called, and questions not asked
38. At various stages each side made a point that the other side had access to an individual who, it was contended, could have given better evidence on a point than that party had advanced, and suggested I should draw a negative inference. This is a legitimate submission and can be very telling in a specific instance. However in the end both sides were able to make very much the same points, which were generic in nature. For example while Illumina appears to have access to Prof Burgess who could give more direct evidence about the work published in Metzker (for example) since it came from his group but was not called, so it turned out MGI has access to Dr Metzker himself but did not call him either. There was also a suggestion that Prof Church, some of whose papers were in the case, could have been called to say more by MGI or could have been asked about more by Illumina. It is relevant to bear in mind that the court always controls expert evidence and the Patents Court in particular is astute to restrict overlapping expert evidence and to encourage cross-examination which is focussed only on the major issues. This general approach to case management means that one cannot assume a party always felt free to call further experts or to ask further questions. In the end I have decided to decide this case as best I can based on the evidence that is here, of which there is a lot, rather than speculating about why there is not even more evidence.
The modified nucleotide patents -  578,
578, 289 and 433
289 and 433
39. In order to make sense of what follows, it is necessary to understand some of the technical background and how MGI’s obviousness case is put.
40. In the 1970s two ways of sequencing DNA were devised, each named after their inventors. They are Maxam-Gilbert sequencing and Sanger sequencing. Maxam-Gilbert sequencing is based on cutting the DNA strands using reagents which break the sequence at known places and analysing the results to deduce the original sequence. The Sanger sequencing technique is different and supplanted Maxam-Gilbert sequencing. Automated machines running Sanger sequencing were used in the human genome project in the 1990s. Sanger sequencing is described in the Primer. Starting from the double stranded DNA of interest, a single strand is taken and used as a template in the method. DNA polymerase is used to add complementary nucleotide bases to the single template strand, one at a time. The complementarity of DNA means that the particular nucleotide base added at a given stage by the polymerase enzyme will be determined by the template sequence. So if the relevant nucleotide in the template is G then a C will be added to the growing complementary strand (G and C pair with one another). The trick to Sanger sequencing is that the free nucleotides to be added are not in their natural form.
41. Natural nucleotides have the capacity to form chains by joining together. Each nucleotide has a chemical group at the 5' position and another at the 3' position. The chemical group at the 3' position is a hydroxyl (OH) group and the group at the 5' position is a triphosphate ester. The two ends connect together to form a link in the chain called a phosphodiester bond, liberating a molecule called pyrophosphate. A single strand of DNA therefore consists of a chain of these nucleotides and will have a “3' end” at one end of the chain and a “5' end” at the other end of the chain.
42. The way natural DNA synthesis works is that when a new nucleotide is added to the complementary strand, its 5' end is linked to the 3' end of the existing nucleotide which was already present. Once incorporated the unused 3' end of that newly linked nucleotide is ready to connect to the next fresh nucleotide, and so the complementary chain will grow.
43. In Sanger sequencing the new nucleotides are not natural because their 3' ends lack the 3' hydroxyl group. In the relevant naming convention it is called a dideoxynucleotide triphosphate (ddNTP). The reason for that name is that one starts conceptually at RNA. That consists of nucleotides called ribonucleotides (because they consist of a nucleobase and a ribose sugar moiety). It is Ribo-Nucleic-Acid. Then one has DNA, which is Deoxy-ribo-Nucleic-Acid because each sugar lacks one of the oxygens (at the 2' position) found in ribose. Then if one knocks off the 3' hydroxyl as well, that produces a di-deoxy-ribo-nucleotide because it lacks two of the oxygens found in the reference ribose structure.
44. So in Sanger sequencing when a ddNTP is added, the chain cannot grow any further. Since there are four nucleotides (C, G, A and T) one can make four mixtures whereby each mixture has all four of C, G, A and T nucleotides in it but in each mixture, some examples of one kind of nucleotide are in the blocked ddNTP form instead of the natural dNTP form. Therefore when the DNA polymerase incorporates a ddNTP into the newly synthesised DNA strand, synthesis of the strand ceases (i.e. chain termination occurs). The Sanger sequencing process therefore results in the synthesis of a large number of copies of the template strand, which are terminated at random lengths according to the position at which a ddNTP is incorporated. A population of DNA strands of different lengths is therefore obtained, which end either with A, T, G, or C. These DNA strands of different lengths are then resolved using manual or automatic approaches and the DNA sequence can be understood. In a manual version of the process radiolabelled ddNTPs are used and the resulting radiolabelled copies of the template strand are size separated by gel electrophoresis. The DNA sequence of the template strand is determined from the order of the bands in the gel, as shown below:
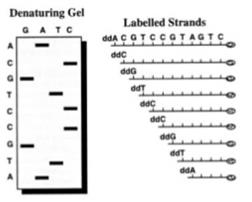
45. However despite its widespread use, Sanger sequencing has drawbacks. Attempts were made to improve the Sanger method itself and to find different, better ways of sequencing DNA.
46. So far the material in this background section would also be common general knowledge. What follows from here is not necessarily common general knowledge.
47. An expression used a lot in this case, and found in the patents, is “sequencing by synthesis” (SBS). It does not have a precise definition but it is a useful term nevertheless. From the explanation above Sanger sequencing involves synthesising a new strand of DNA and so in that sense it could be said to be a form of sequencing by synthesis. However not all methods of sequencing involve synthesis. For example Maxam-Gilbert sequencing is not sequencing by synthesis.
48. The DNA sequencing technique in issue in this case can be called sequencing by synthesis using reversible chain terminators (RCTs). In Sanger sequencing the ddNTP blocks any further synthesis of the complementary DNA strand. The ddNTPs are chain terminators. However well before the 2002 priority date some in the art had the idea of trying to do a kind of Sanger technique but with a chain terminator which was reversible. If the blockage could be reversed after the identity of the added nucleotide had been confirmed, then the next nucleotide in the chain could then be added and the process repeated.
49. The Metzker and Canard papers mentioned already relate to attempts, published in the 1990s, to make sequencing by synthesis using reversible chain terminators work. The precise state of sequencing by synthesis using reversible chain terminators by that date is disputed but, looking ahead and as explained below, by 2002 the technique had not been shown to be work in a useful way.
50. Briefly put, the invention(s) claimed in the modified nucleotide patents are concerned with using an azidomethyl group as a reversible chain terminator in sequencing by synthesis. I have not forgotten the insufficiency issues, which will be addressed in context, but in any event it is clear today that sequencing by synthesis using an azidomethyl group as the reversible chain terminator works.
51. This is sufficient technical background to understand how MGI puts it case on obviousness. As pleaded MGI relied on Tsien, Ju and two Zavgorodny papers. A vital difference between the Zavgorodny papers on one hand and the Tsien and Ju references on the other is that while Tsien and Ju are about sequencing by synthesis using reversible chain terminators, the Zavgorodny papers are not.
52. The Zavgorodny papers do describe an azidomethyl blocked nucleic acid molecule but they are not concerned with DNA sequencing at all (there are other reasons for using blocking groups). Whereas Tsien and Ju describe sequencing by synthesis using reversible chain terminators but do not include any reference to azidomethyl as a reversible chain terminator group. Now by the start of the trial MGI had abandoned the case over Tsien or Ju and concentrated on the case over Zavgorodny. However it is manifest that a skilled person who had never heard of the technique of sequencing by synthesis using reversible chain terminators, and who read either Zavgorodny paper in 2002, could not possibly think of the invention because nothing in either paper would prompt someone who had no knowledge of sequencing by synthesis to think of the technique at all.
53. MGI’s primary case is that the skilled person for the purposes of obviousness is or includes a team interested in or researching sequencing by synthesis using reversible chain terminators. MGI says that, based on this definition of the team, it follows that the skilled person will have sequencing by synthesis using reversible chain terminators in mind when reading the Zavgorodny reference. That does not necessarily mean the invention has to be obvious, but without it, MGI’s primary case would be untenable.
54. Illumina contends that MGI’s primary case is bound to fail because it is based on an illegitimate definition of the person skilled in the art, given the failure as Illumina sees it, of reversible chain terminators by the priority date. Illumina contends the true definition of the person skilled in the art in this case is a team interested in developing improved methods of sequencing, which would include improvements to Sanger sequencing. On Illumina’s case that team was unaware of sequencing by synthesis using reversible chain terminators. To that team (says Illumina) the invention is not obvious over Zavgorodny.
55. That leads to MGI’s alternative case, which is based on Illumina’s definition of the skilled team. It is said that sequencing by synthesis using reversible chain terminators would be part of the common general knowledge of that skilled team. They would have it firmly in mind if Zavgorodny had crossed their desk in 2002.
56. However the problem with this alternative case is that, for the skilled team as defined by Illumina, the idea of sequencing by synthesis using reversible chain terminators was not part of the common general knowledge. There is clear evidence (and I find) that real skilled people focussed on improvements to Sanger sequencing had never heard of it. (The fact that some such people may well have heard of it does not make it common general knowledge.)
 57.
57. style='font:7.0pt "Times New Roman"'> Therefore the width of the correct definition of the person skilled in the art is a vital issue in this case and that is the next question.
style='font:7.0pt "Times New Roman"'> Therefore the width of the correct definition of the person skilled in the art is a vital issue in this case and that is the next question.
The skilled person
58. Who is the person skilled in the art? Stated generally the law is clear that patents are directed to those likely to have a real and practical interest in the subject matter of the invention. This language is based on paragraph 81 on the judgment of Henry Carr J in Garmin v Philips [2019]  EWHC
EWHC 107 (Ch) in which the judge summarised the law in this area. The real practical interest in the subject matter includes devising the invention itself as well as putting it into practice and so, as was highlighted in Schlumberger v EMGS [2010] EWCA Civ 819, the concept of the person skilled in the art actually applies in two distinct circumstances. In a proper case they may be two different persons (or teams). One person skilled in the art is the person to whom the patent is addressed and whose attributes, skills and common general knowledge will be necessary to implement the patent. As Illumina submitted that person is always going to be the appropriate skilled team from the point of view of addressing sufficiency, since the patentee is entitled to put together his invention by combining any skill-sets he likes. As Pumfrey J said in Horne Engineering v Reliance Water Controls [2000] FSR 90 (quoted in Schlumberger at para 51)
107 (Ch) in which the judge summarised the law in this area. The real practical interest in the subject matter includes devising the invention itself as well as putting it into practice and so, as was highlighted in Schlumberger v EMGS [2010] EWCA Civ 819, the concept of the person skilled in the art actually applies in two distinct circumstances. In a proper case they may be two different persons (or teams). One person skilled in the art is the person to whom the patent is addressed and whose attributes, skills and common general knowledge will be necessary to implement the patent. As Illumina submitted that person is always going to be the appropriate skilled team from the point of view of addressing sufficiency, since the patentee is entitled to put together his invention by combining any skill-sets he likes. As Pumfrey J said in Horne Engineering v Reliance Water Controls [2000] FSR 90 (quoted in Schlumberger at para 51)
“it is often possible to deduce the attributes which the skilled man must possess from the assumptions which the specification clearly makes about his abilities.”
59. The second kind of skilled person is the one relevant to obviousness. In nearly all cases they will be the same as the first kind (Schlumberger para 40) but Schlumberger was a case in which they were not, and that case illustrated why it would have been wrong to treat the two kinds as necessarily the same. The question then is what are the legal principles which define the identity of the second kind of skilled person.
60. One principle in Schlumberger was identified in paragraph 65:
“In the case of obviousness in view of the state of the art, a key question is generally “what problem was the patentee trying to solve?” That leads one in turn to consider the art in which the problem in fact lay. It is the notional team in that art which is the relevant team making up the person skilled in the art.”
61. This will be the governing approach in many cases but it can lead to trouble. There are cases of so called “problem-inventions” in which simply asking if the solution is obvious given the problem is unfair because inventiveness lay in identifying the problem. The fact the solution was obvious once you identify the problem does not prove a lack of inventive step in such a case. In fact experience shows that real cases are often more nuanced in that there can be aspects of a problem which are not common general knowledge and so one cannot always draw a sharp line between problem invention cases and other cases.
62. Furthermore, blindly applying an approach based on the definition of the problem to be solved could lead to a very narrowly defined skilled person and that can create its own difficulties, which were well described by Peter Prescott QC in Folding Attic Stairs v The Loft Stairs Company Ltd [2009]  EWHC
EWHC 1221 (Pat). He showed why it could be wrong to frame the art in a narrow way. At paragraphs 33-34 he said:
1221 (Pat). He showed why it could be wrong to frame the art in a narrow way. At paragraphs 33-34 he said:
“33. Common general knowledge is quite different. It is what people skilled in the art actually do know, or ought to know, provided that knowledge is regarded as sound. Common general knowledge is not a phrase used in the Patents Act or the European Patent Convention. It would be difficult to define the person skilled in the art in this case, or the common general knowledge, because so far as I know there is no recognised profession or calling of designing folding attic stairways. At the date of the patent nobody seems to have done it in the British Isles except the Claimant and perhaps one other company. There must have been one or more companies in America, I suppose. It is unfair to define an art too narrowly, or else you could imagine absurd cases e.g. “the art of designing two-hole blue Venezuelan razor blades”, to paraphrase the late Mr T.A. Blanco White. Then you could attribute the “common general knowledge” to that small band of persons who made those products and say that their knowledge was “common general knowledge” in “the art”. That would have the impermissible result that any prior user no matter how obscure could be deemed to be common general knowledge, which is certainly not the law.
34. However it does not make much difference in this case, because the amount of special knowledge that is required to understand the patent in suit is not great. I would identify the person skilled in the art as one who has practical experience as a manufacturing carpenter, assisted by a metal fabricator. At the date of the patent (1996) this person or team would be vaguely aware of folding stairways in general terms, at most. The actual construction of old Stira, while known to many customers, was not common general knowledge in the art, in my judgment.”
63. So while Folding Attic Stairs neatly explains one of the difficulties, given its facts the judge did not have to identify a principle to be applied to solve it. Furthermore, while a too narrow definition could be unfair to the inventors, it could be just as wrong and unfair to the public to define a team so widely that their common general knowledge is so dilute as to make something seem less obvious than it really was (see Pumfrey J in Mayne v Debiopharm [2006]  EWHC
EWHC 1123 (Pat) at paras 3-4).
1123 (Pat) at paras 3-4).
64. The other principled approach from Schlumberger to identifying the second kind of skilled person is to look at what is really going on in the art up to and at the priority date (Jacob LJ paragraph 42):
“I think one can draw from [Dyson v Hoover] that the Court, in considering the skills of the notional “person skilled in the art” for the purposes of obviousness will have regard to the reality of the position at the time. What the combined skills (and mind-sets) of real research teams in the art is what matters when one is constructing the notional research team to whom the invention must be obvious if the Patent is to be found invalid on this ground.”
65. This was summarised in Medimmune v Novartis [2012] EWCA Civ 1234 at paragraph 76-77 as a principle that the court will have regard to the reality of the position at the time and the combined skills of real research teams in the art. In Medimmune the court found that “antibody engineering” was an established field by the priority date. There were 10 such real teams in the evidence and they were all likely to have a practical interest in the subject matter and to have the skills to implement it.
66. In the present case Illumina proposed, based on Medimmune, that a sensible test was to require something which could properly be called an established field at the priority date. Depending on the facts the field could be a research field as in Medimmune or a field of manufacture as in Folding Attic Stairs.
67. The advantage of this test is that it provides a principled way of solving the problem identified in Folding Attic Stairs. If the design and manufacture of folding attic stairs in particular was an established field then there is nothing unfair in defining the skilled person that way. But if not then the wider definition (general carpenter plus metal fabricator) is appropriate. In other words the width of the field in which the skilled person operates for the purposes of obviousness (aka the “art in which the problem lay” (per Schlumberger)) is ultimately governed by what was actually going on up to the priority date. It is not primarily a function of the invention itself, the problem to be solved, nor the patent’s text.
68. I conclude that in a case in which it is necessary to define the skilled person for the purposes of obviousness in a different way from the skilled person to whom the patent is addressed, the approach to take, bringing Schlumberger and Medimmune together, is:
i) To start by asking what problem does the invention aim to solve?
ii) That leads one in turn to consider what the established field which existed was, in which the problem in fact can be located.
iii) It is the notional person or team in that established field which is the relevant team making up the person skilled in the art.
69. Sub-paragraph (i) is phrased as it is rather than referring to a problem the patentee was trying to solve, because although those words are in Schlumberger, I do not believe the Jacob LJ was there intending to suggest that the identification of the problem is anything other than an objective exercise.
70. Sub-paragraph (ii) is phrased as it is for two reasons. First, there always will be some established field in which the problem would have been located. How wide the definition of that field should be will depend on the facts and what was going on in reality. Second, the field is the one in which the problem can be located, looking back from today as an exercise in hindsight. It does not matter at this stage if those in that field at the priority date did not perceive the particular problem or did not perceive it in the manner it is now characterised.
71. Finally I will say something about the evidence. There was a dispute at the outset of the trial about an aspect of MGI’s case relating to the definition of the skilled person. Part of MGI’s skeleton advanced a different skilled person from the one in Prof Marx’s evidence. Related to this, in his fifth report, the professor sought to clarify something he had said earlier on this topic which Illumina contended was in fact a shift, related to the same point. It is necessary for experts to explain who they think the skilled person is, not least in order to explain the basis on which they are giving their evidence. However while the expert and other evidence is critical to resolving a dispute about the identity of the skilled person, in the end the identity of that person is a matter for the court, applying the law to the facts to reach a conclusion.
Person skilled in the art– the facts
72. I start with the 2002 priority date and the modified nucleotide patents.
73. Prof Leadlay’s view was that the skilled person to whom the patents were addressed was an individual or a team involved generally in the development of DNA sequencing methods. The skilled person would have knowledge of molecular biology and genetics and would likely have a masters or doctorate in biochemistry, molecular biology, genetics or organic chemistry, as well as several years’ experience relating to DNA sequencing.
74. Prof Marx essentially agreed with Prof Leadlay, save that his view was that the methods the skilled team would be concerned with developing included sequencing by synthesis methods. Prof Winssinger gave evidence about this which overlapped with Prof Marx and added nothing to it. Prof Marx’s view was that there were a number of groups actually involved in sequencing by synthesis at the priority date.
75. In his fifth report Prof Marx was asked by MGI to clarify what he meant by “methods including sequencing by synthesis". He said he did not think that teams involved in or interested in developing other methods of sequencing but not sequencing by synthesis would be interested in the teaching of the patents. This was not really a clarification. It was a shift in position. And while no doubt Prof Marx was unaware of this, it was obviously driven by a shift in thinking by MGI’s legal team as they decided to drop Tsien and Ju and concentrate on Zavgorodny.
76. Thus the essential difference between the parties was whether the skilled person is to be defined by reference to sequencing by synthesis or not and I turn to address that.
77. The problem which the invention claimed in the modified nucleotide patents aims to solve can be stated in different ways. MGI contended that based on the disclosure of the patents, the problem the patentee was trying to solve was the identification of removeable protecting groups which could meet the requirements for use in methods of sequencing by synthesis; in other words, to find improved removable protecting groups to act as reversible chain terminators in sequencing by synthesis. Illumina’s formulation was not very different. It was the identification of a successful reversible blocking group for the 3' position for use in sequencing by synthesis. At this stage nothing turns on the differences between these formulations, nor does it follow that the skilled person, however defined, has that problem in mind. That latter question depends on the common general knowledge.
78. The next question therefore is to examine what the established field was in which this problem would be located. The parties’ submissions were far apart but in my judgment the evidence by the end of the trial was tolerably clear.
79. By the priority date there was a large body of skilled people interested in improvements to DNA sequencing in general. One known area where improvements would be of real interest was in improvements to Sanger sequencing. People with a particular interest in improving Sanger sequencing were not focussed on trying to find new techniques, they were trying to optimise the known and very successful method. Many would not have heard of the idea of reversible chain terminators.
80. As Prof Marx explained in his report, the term sequencing by synthesis was not in universal use at the priority date. For what it is worth I am sure anyone involved in DNA sequencing would understand what it meant if they heard it but that is different from the term itself being in general use. The expression sequencing by incorporation is another phrase used at the time which conveys a similar sense. Today the term sequencing by synthesis does not generally include Sanger sequencing. Another expression used now but not then is next generation sequencing.
81. However by the priority date there were a number of real teams researching alternatives to Sanger sequencing. These included techniques which one would now call sequencing by synthesis. By 2002 one (and only one) such technique had just been demonstrated to work and an early machine which worked that way was available. The technique is called pyrosequencing.
82. Pyrosequencing works because the pyrophosphate released as a nucleotide is incorporated into a complementary strand can be detected by generation of light. The technique uses natural dNTPs. The way it works is that the growing strand is exposed to a single type of dNTP at a time - C, G, A or T. You will know which of those has been added to the strand because when that happens the pyrophosphate is released and that can be detected by generation of light. Once the pyrophosphate is detected, a solution of a different dNTP is added and the process repeated. A drawback is that as described one cannot tell the difference between adding a single nucleotide and adding two or more of the same nucleotide. In other words it is vulnerable to repeats. In fact there is a way of addressing that by measuring the intensity of the light flash generated following pyrophosphate release but that is not relevant.
83. Although pyrosequencing was the most advanced alternative to Sanger at 2002, there were other techniques which were being considered in the years up to and including the priority date. One of those was the use of reversible chain terminators in what is now called sequencing by synthesis. MGI referred to a number of groups with an interest in reversible chain terminators, over and above Solexa itself.
84. There was a research group at Baylor College of Medicine (Richard Gibbs) and Texas A&M (Kevin Burgess). This group published five papers on reversible chain terminators from 1994 until 1999 including the Metzker 1994 paper in Nucleic Acids Research. Illumina submitted the work of this group had petered out by the priority date, and the evidence of a later, post priority grant application did not show that the group continued but rather was a consequence of the later developments. The evidence about this issue was thin. It is more likely than not that the focus of this group on reversible chain terminators after the 1999 did wane. I am not convinced the grant application showed that the work continued in the intervening years, rather it showed that workers with a real interest in the subject encountered a reason to get going again.
85. There was a research group at the Pasteur Institute in Paris led by Bruno Canard and Robert Sarfati. This group had published six papers from 1994 to 1999 including a 1994 Canard paper in Gene and a 1995 Canard paper in PNAS. They also filed a patent (Canard 5,798,210) which related to sequencing by incorporation and included reference to reversible chain terminators.
86. There was a group led by Prof Ju at Columbia University. Their work led to the Ju patent application cited as prior art by MGI in this case. It claimed priority from a US filing in 2000 and was published in April 2002. The abstract provides:
“This invention provides methods for attaching a nucleic acid to a solid surface and for sequencing nucleic acid by detecting the identity of each nucleotide analogue after the nucleotide analogue is incorporated into a growing strand of DNA in a polymerase reaction. The invention also provides nucleotide analogues which comprise unique labels attached to the nucleotide analogue through a cleavable linker, and a cleavable chemical group to cap the -OH group at the 3' -position of the deoxyribose.”
87. The idea of the cleavable chemical group on the deoxyribose referred to in this passage is the same thing as a reversible chain terminator. The Ju group published their first two papers in 2003 in PNAS (one with a first author Zengmin Li and the other with a first author Xiaopeng Bai). They were submitted before the 2002 priority date. The Li paper reports the results of experiments using photocleavable linkers to attach fluorophores to the nucleotides in sequencing by synthesis approach. It gives 1988 as the date when the concept of sequencing by synthesis was revealed (in a paper by Hyman), it refers to pyrosequencing and also refers to various reversible chain terminator papers such as Metzker 1994.
88. The company Genovoxx based in Lübeck filed a patent application (WO 02/088382) claiming priority from 2001 and published in November 2002. It relates to sequencing by synthesis using reversible chain terminators. Prof Church (see below) had been in contact with that group after the priority date. Illumina positively rely on Genovoxx’s work as pointing in a quite different direction from the invention in issue and I will address that below. The point at this stage is that this group was clearly active in this area at the time.
89. Other companies who applied for patents relating to sequencing by synthesis using reversible chain terminators before the priority date were Medical Biosystems Ltd based in Totnes (application published in 1999); Caliper Technologies Corp of Mountain View, California (application published in 2000); ASM Scientific Inc. of Cambridge, Mass. (application published in 2000), Illumina itself prior to acquiring Solexa (application published in 2000); Amersham Pharmacia Biotech (application published in 2001); and Agilent Technologies Inc. of Palo Alto (application filed May 2002, post published).
90. There is no need to go into further detail about any of these other companies at this stage. As with Genovoxx, Illumina points out that the approaches some of these companies appeared to be taking is in a different direction from the invention in issue but as I have already said, that is not germane at this stage. It is also important not to read too much into the fact that a company has filed a patent application. It does not, for example, prove that that company has done any active “wet chemistry”. Nevertheless in the context of the other evidence in my judgment the totality of these patent applications support the point MGI seeks to make.
91. Prof Church in his evidence listed a number of groups and individuals with whom he had personal dealings who, he said, would have known about reversible chain terminators. A number of them have been mentioned above. That is not the same thing as saying that groups were carrying out active research on the topic - or even thinking about it. His evidence is more directly relevant to common general knowledge.
92. Standing back, it cannot be said that sequencing by synthesis or sequencing by synthesis using reversible chain terminators was as established as antibody engineering in the Medimmune case. For example there is no evidence of job advertisements seeking “sequencing by synthesis” engineers nor are there textbooks or conferences specifically direct to the topic. (The conference in 1994 put to Prof Leadlay was not one.) However it is clear and I find that a wide range of independent scientific groups, both academic and in industry were interested in and looking directly at this area. This state of affairs had existed for some years. The fact that sequencing by synthesis using reversible chain terminators had not succeeded by the priority date is highly relevant but not determinative. By 2002 there was live interest from a number of people, and real research was underway at independent centres. Papers were being published by groups of workers, referring to the work of others. As a discipline in its own right, distinct from Sanger sequencing, sequencing by synthesis was at a nascent research stage, with pyrosequencing the most advanced technique.
93. I find that sequencing by synthesis was an established field of research by 2002. Looking at it another way, having regard to the depth of work published by some groups, and the wide variety of groups with an interest in the area (which interest by 2002 was made public), it would be wrong to approach the validity of a patent about sequencing by synthesis with a 2002 priority date as if the skilled person was a DNA sequencing generalist without an interest in sequencing by synthesis.
94. I would say the right level of generality to describe the established field of research is sequencing by synthesis, thereby including at least pyrosequencing, rather than sequencing by synthesis using reversible chain terminators in particular.
95. This conclusion explains why highly knowledgeable and experienced individuals in DNA sequencing in general, such as Prof Leadlay, Prof Mattick and Prof Lovett, had not heard of reversible chain terminators at the priority date. They were not focussed on sequencing by synthesis at that time. At the risk of repetition, this does not mean Prof Leadlay (or Prof Marx) does not have relevant evidence to give in this case because the general area in which the skilled person is interested is DNA sequencing.
96. Therefore the person skilled in the art at the 2002 priority date, at least for the purposes of considering obviousness, is a team working on research into sequencing by synthesis.
97. MGI referred to a number of documents from proceedings in other jurisdictions (including the EPO) relating to this European patent, or patents in the same family, in which Illumina had characterised the skilled person in a manner similar to the way MGI put its case here and not as Illumina submitted to me. Illumina were free in this jurisdiction to try and prove something different from that in other jurisdictions, with different evidence. However I have found that attempt fails.
98. The skills involved were addressed by Prof Leadlay and Prof Marx in slightly different ways but I do not believe any of the distinctions between them amounted to a material dispute. I find that the team can be regarded as having two members. One member would have a background in molecular biology or genetics, with a focus on DNA sequencing in particular, the other member would have a background in organic chemistry. They would both have a post-graduate degree, probably a PhD but perhaps a Masters, and some years research experience.
99. In fact having defined the team this way, it would be the same skilled team to whom the patent is addressed and who would be relevant for sufficiency and all other issues.
100. Looking ahead to the other patents in this case (412 and 415), the definition of the skilled person at the relevant dates for those patents - 2004 and 2006 will be the same as for 2002. An additional member of the team would be a fluorescence chemist but that is not relevant to the modified nucleotide patents.
The common general knowledge
101. The classic statement of the law on common general knowledge is in General Tire v Firestone [1972] RPC 457 at p. 482. More recently, the Court of Appeal in Idenix v Gilead [2016] EWCA Civ 1089 at para 72, citing General Tire, summarised the correct approach to common general knowledge as follows:
“It follows that the common general knowledge is all that knowledge which is generally regarded as a good basis for further action by the bulk of those who are engaged in a particular field. It is that knowledge which those working in that field will bring to bear when they are reading or learn of a piece of prior art. It is not necessary that those persons have that knowledge in their minds, however. The common general knowledge includes material that they know exists and which they would refer to as a matter of course if they cannot remember it and which they understand is generally regarded as sufficiently reliable to use as a foundation for further work”.
102. A point arises on the principles. The reference to a “good basis for further action” does not mean only things which work can be common general knowledge. The common general knowledge of a skilled person will often be as much about knowing what does not work as it is about knowing what does. Both are examples of a “good basis for further action” in that they are ideas which are worth acting upon. In a similar vein, in Merck v Ono [2015]  EWHC
EWHC 2973 (Pat) at para 24, I held that the common general knowledge includes contradictions as long as the information was sufficiently well known to be common general knowledge. So the fact a given technique was something which had been proposed for some years, tried out by a number of groups, but not (yet) shown to work, would not in and of itself preclude information about that technique being held to be part of the common general knowledge. A technique like that which was sufficiently well known could be common general knowledge.
2973 (Pat) at para 24, I held that the common general knowledge includes contradictions as long as the information was sufficiently well known to be common general knowledge. So the fact a given technique was something which had been proposed for some years, tried out by a number of groups, but not (yet) shown to work, would not in and of itself preclude information about that technique being held to be part of the common general knowledge. A technique like that which was sufficiently well known could be common general knowledge.
Common general knowledge - the facts
103. A basic introduction to Sanger sequencing, which was part of the common general knowledge, was set out above as technical background. In fact the common general knowledge of the members of the skilled team would involve a much more sophisticated appreciation and understanding of matters of that kind. However most of the detailed knowledge of molecular biology, biochemistry and organic chemistry which the team would possess as a matter of common general knowledge is not relevant to the issues and does not need to be addressed here.
104. A team working on research in sequencing by synthesis at the relevant date would be well aware of pyrosequencing. It was becoming an established technique. However in my judgment the common general knowledge would also include knowledge of the concept of reversible chain termination. A fair number of papers related to reversible chain terminators had been published before the priority date. As a matter of the common general knowledge of a sequencing by synthesis skilled team, the team would know that there was a body of papers and know how to find them. I would hold that unprompted, their common general knowledge would include knowledge of the existence of two particular groups who had published experimental results in more than one paper. They were the Gibbs/Burgess group and the Sarfati group. Again, unprompted, the common general knowledge would include the existence of two particular papers, which were frequently cited. They are Metzker 1994 and Canard 1994. This is not a finding that any particular content in either paper was common general knowledge. What was common general knowledge was that these papers existed, published results and represented the farthest anyone had got with reversible chain terminators as a concept.
105. Therefore to correctly characterise the common general knowledge of reversible chain terminators depends on taking a look at these papers.
Metzker 1994
106. The authors call their proposed technique BASS (Base Addition Sequencing Scheme). It is sequencing by synthesis using reversible chain terminators. The major potential advantages of the technique over Sanger sequencing are mentioned. They are: no need for gel electrophoresis to resolve bases, and tremendous capacity for simultaneous analysis of multiple samples.
107. In his first report Professor Leadlay summarised the technical content of the Metzker 1994 paper. I accept his summary, from which much of what follows is based. The authors prepared eight dNTPs with seven different types of 3' modification. Each modification put a different protecting group on the 3' oxygen. One group tested was a methyl and that was used for two bases (hence eight dNTPs with seven modifications). They conducted a series of experiments they called termination assays. These were an attempt at one cycle of stop start DNA synthesis using a DNA template. The eight dNTPs were tested with eight different DNA polymerases. The results are tabulated in table 2. Of the seven types of 3' modification tested, only three of them (3'-O-methyl, 3'-O-allyl, and 3'-O-(2-nitrobenzyl)) showed termination activity. The other types of 3' modification tested in the termination assay either showed no termination activity or caused inhibition of the polymerase.
108. The authors also reported that with a 2-nitrobenzyl protecting group on the 3' oxygen, they were able to cleave it off photolytically (the 2-nitrobenzyl group is sensitive to UV). They were also able to incorporate a further nucleotide into the chain in that case albeit the nucleotide added was a natural one, rather than a modified nucleotide. In other words Metzker 1994 achieved a single cycle of deprotection and re-initiation of DNA synthesis using 3'-O-(2-nitrobenzyl)-dATP.
109. Prof Marx summarised Metzker reporting a full cycle (of incorporation, deprotection and reinitiation of DNA synthesis) for the 3'-O-(2-nitrobenzyl) blocked nucleotides. I accept that subject to the qualification that the subsequent incorporation was of a natural dNTP.
110. This paper does not describe an experiment using a nucleotide ligated to a detectable label such as a fluorophore. The detection used is radiolabelling which does not distinguish between different nucleotides.
Canard 1994
111. The aim of the work reported in Canard 1994 was to design 3'-modified dNTP substrates for DNA polymerases, such that the 3'-moiety would be different for each base G, A, T or C, be easily identified e.g. by fluorescence, and be removed under conditions compatible with DNA stability to restore an unprotected 3'-hydroxyl end. The differences between Canard 1994 and Metzker 1994 are that a different 3' blocking group was used from that in Metzker 1994, and fluorescent labels were used to distinguish different nucleotides. The fluorescent labels were fixed to the blocking group directly. A single cycle of incorporation was reported. The method was said to work with three more DNA polymerases, albeit the data was not shown for those. The fact the data was not shown (in that era of scientific publication before the ready availability of further data via the internet) does not mean a skilled person would simply ignore what is said.
112. There was also a Canard 1995 paper but it was not concerned with reversible chain terminators.
Later papers (1999)
113. The last paper before the priority date from the Sarfati group which had published the Canard papers was Rasolonjatovo 1999. This reported incorporation with another 3' blocking group but not chain termination. The paper ends by saying that further studies are in progress.
114. The last papers before the priority date from the Gibbs/Burgess group, which had published the Metzker papers, were two papers in 1999 with Welch as lead author. One in the Journal of European Chemistry refers to combinatorial DNA sequencing. It reports tests using photolabile 2-nitrobenzyl 3' blocking groups and concludes (in the abstract) that both nucleoside triphosphates and the DNA polymerase enzyme must be modified if the proposed technique is to be viable. The other, in the journal Nucleotides and Nucleosides, notes that several groups have been interested in the technique this group calls BASS. The paper reports tests with two photolabile 2-nitrobenzyl 3' blocking groups but they do not achieve incorporation. Further experiments are in progress.
Other kinds of sequencing by synthesis
115. It is convenient at this stage to mention another sequencing by synthesis paper published in 1999 albeit not one about reversible chain terminators. It is a paper in Nucleic Acids Research by Professor Church and Dr Mitra which proposed a new technique using numerous PCR colonies or “polonies”. This included the suggestion of using a high throughput sequencing method such as pyrosequencing but also described a new sequencing by synthesis idea called FISSEQ (fluorescent in situ sequencing extension quantitation). This technique would employ fluorescently labelled nucleotides. They would not be blocked. As in pyrosequencing, a buffer with one type of nucleotide would be added at a time to a system with the DNA template strand and polymerase. Therefore when the buffer added contained the type of nucleotide which was added to the growing strand, incorporation would be detectable by fluorescence and the cycle repeated. Like pyrosequencing this is vulnerable to repeats but also like pyrosequencing, as Prof Church himself explained, intensity could be used to try to count the number of nucleotides incorporated.
116. Two papers concerning pyrosequencing were also referred to in the evidence, Ronaghi 1997 and Nordstrom 2000. They cross-refer to the work of Metzker without explaining what it is. If they are relevant at all these papers provide a further indication in support of the idea that sequencing by synthesis as a whole was an established field.
117. Other ideas for alternatives to Sanger sequencing were mentioned in the literature before 2002. Some are mentioned in a paper by Marziali but it is not necessary to grapple with them. This is not a case in which the availability of other alternative ways forward plays an important part in the analysis.
The evidence as a whole about the state of reversible chain terminator in the common general knowledge
118. To the skilled person in 2002, the work of the two groups, Gibbs/Burgess and Sarfati, were by far the most well advanced in relation to reversible chain terminators.
119. MGI emphasised that in cross-examination Prof Leadlay explained that Metzker 1994 was showing a single cycle, which he described as very, very preliminary work, and said the same comment applies to Sarfati (T2/19911-24). MGI pointed to the text in Canard 1994 that explained that “our results show that it is possible to reach high incorporation levels required to perform several cycles in a row” and submitted that Prof Leadlay accepted that Metzker 1994 “sets the stage” and that Canard demonstrated the proof of principle.
120. I accept MGIs submissions but only up to a point. They are more reflective of the view of someone reading these papers in 1994 than they would be by 2002, by which time no further significant steps forward had occurred. By 2002 a more accurate characterisation of the view of the skilled person, as Illumina submitted, was that neither Metzker nor Canard had achieved anything more than an initial incorporation in 1994, and their later efforts up to 1999 had not succeeded.
121. What was the common general knowledge about the problems which had to be solved? MGI’s characterisation of the “problem to be solved” was the identification of a reversible chain terminator which could meet the requirements for use in sequencing by synthesis. A critical question is whether that problem or something like it was part of the common general knowledge of the skilled person. The reason this is critical is that the evidence of Prof Marx that the invention was obvious was based on an approach made clear in his evidence and cross-examination. His approach was that the skilled person looks at Zavgorodny with the specific aim in mind of finding a blocking group he might be able to use in a reversible chain terminator sequencing process. Moreover the questions put to Prof Leadlay were on essentially the same premise (that the skilled person came to the cited art interested in taking forward sequencing by synthesis with a new reversible chain terminator).
122. Illumina submitted that the idea of pursuing new chemical groups as reversible chain terminators on the 3' end of the nucleotide in sequencing by synthesis was not representative of the common general knowledge at 2002. I agree. My reasons are as follows.
123. First, the skilled person did not lack chemical groups to try as protecting groups. The Greene & Wuts textbook illustrates that.
124. Second the papers concerned with reversible chain terminators were not suggesting what was required to overcome their absence of success was to test new chemical groups as reversible chain terminators.
125. Third, as best one can tell from the patent applications filed around the priority date, of the work which independent groups did do at around that time, it did not involve trying new potential reversible chain terminators at the 3' position. One group (Amersham) was interested in making modifications at the 4' position. The other group was Genovoxx. Their approach, based on their patent application, was to avoid pursuing a 3' modification and instead put a sterically demanding group on the base to prevent incorporation. Prof Marx accepted that this approach of Genovoxx was a fair reflection of the attitude of those in the art at the time. I infer that Genovoxx were well aware of the earlier proposals to use reversible chain terminators at the 3' position, and did not lack ideas for alternative groups at that location, but took an entirely different approach.
126. Overall, in my judgment the common general knowledge of the skilled person in 2002 was that they knew of the concept of sequencing by synthesis with reversible chain terminators, but they also knew that it had not succeeded in practice. The skilled person also understood that to make it work one needed to come up with a system in which one could repeatably incorporate nucleotides linked to specific labels one at a time in a reversible way, but they did not know with any degree of specificity what particular problem or problems had to be solved so as to take this forward. It may well have been that the technique simply could not be made to work. The attitude of the skilled person in 2002 was not an upbeat one.
127. Prof Church’s explained in his evidence that his view at the time was that sequencing by synthesis would work and just needed the right reversible chain terminator. I do not accept that that view represented the common general knowledge of the person skilled in the art. I think Prof Church may well be making a slip in attributing a view he later had to an earlier time but even if he was not, he is not representative of a person of ordinary skill in the art.
Organic chemistry
128. Two aspects of organic chemistry need to be considered. First it is worth bearing in mind that the idea of a reversible protecting group in organic chemistry is very well established. They find utility throughout organic chemistry as way of protecting one functional group in a molecule from reacting so that changes can be made elsewhere. The Greene & Wuts textbook which has already been mentioned is a large encyclopaedia of possible protecting groups. It includes azides.
129. Second the azide group (three nitrogens in a row) is a well known chemical group. They were known to be cleavable. By 2002 azides had come to some prominence in biochemistry in general as a result of the work of Prof Carolyn Bertozzi in California. The Bertozzi group had used methods referred to as Staudinger chemistry, exploiting a selective chemical reaction between an azide and a phosphine, to produce a stable covalent linkage to join two biological moieties. They specially engineered a phosphine which, on reducing the azide by the Staudinger reaction, trapped the resulting amine in an amide bond thereby linking the two moieties. They demonstrated that this so-called 'Staudinger ligation' could be performed on the surface of cells (Bertozzi 2000) and on intracellular proteins (Bertozzi 2002).
130. The skilled person would know of this work but I am not satisfied it would play any material part at all in their thinking on any relevant issue. It is only hindsight which makes any relevant analogy between this Bertozzi work and the issues in the present case.
The specifications of the modified nucleotide patents
131. As Illumina did in its opening skeleton, I will address the specification of the 289 patent. In fact there are some extra passages in the specification of the  578
578 patent but nothing turns on them at this stage.
patent but nothing turns on them at this stage.
132. Paragraph [0001] explains that the invention relates to modified nucleotides, modified so as to have a removable protecting group. The invention also relates to polynucleotide sequencing methods and a method for chemical deprotection of the protecting group. This would be understood by the skilled person as a reference to reversible chain terminators.
133. Paragraph [0004] states that “sequencing by synthesis of DNA ideally requires the controlled (i.e. one at a time) incorporation of the correct complementary nucleotide opposite the oligonucleotide being sequenced.” It goes on to explain:
i) This allows for accurate sequencing by adding nucleotides in multiple cycles as each nucleotide residue is sequenced one at a time, thus preventing an uncontrolled series of incorporations occurring.
ii) The incorporated nucleotide is read using an appropriate label attached thereto before removal of the label moiety and the subsequent next round of sequencing. In order to ensure only a single incorporation occurs, a structural modification ("blocking group") of the sequencing nucleotides is required to ensure a single nucleotide incorporation but which then prevents any further nucleotide incorporation into the polynucleotide chain.
iii) The blocking group must then be removable, under reaction conditions which do not interfere with the integrity of the DNA being sequenced.
iv) The sequencing cycle can then continue with the incorporation of the next blocked, labelled nucleotide.
v) In order to be of practical use, the entire process should consist of high yielding, highly specific chemical and enzymatic steps to facilitate multiple cycles of sequencing.
134. Paragraph [0005] explains a 3' blocking group is required to prevent the chain from continuing to grow and explains what the ideal features of such a blocking group would be in order to be useful. It must:
i) exhibit long term stability;
ii) be efficiently incorporated by the polymerase enzyme;
iii) cause total blocking of secondary or further incorporation; and
iv) have the ability to be removed under mild conditions that do not cause damage to the polynucleotide structure, preferably under aqueous conditions.
135. Paragraph [0005] refers to these as “stringent” requirements and goes on from paragraph [0006] to describe the problem to be solved, which is that while reversible blocking groups for this purpose have been disclosed previously, none of them meet the criteria above. Reference is made to Metzker 1994 and to the Ju patent application, referring in each case to the use of a 3' allyl blocking group.
136. The specification goes on to present an azidomethyl (-CH2N3) group as a blocking group which meets those stringent requirements. In fact the text also discusses other groups as well but nothing turns on that since the specification clearly discloses azidomethyl as a preferred blocking group and presents results using such a group (e.g. paragraph [0102]). The specification also states in terms at paragraph [0103] that:
“Nucleotides bearing this blocking group at the 3' position have been synthesised, shown to be successfully incorporated by DNA polymerases, block efficiently and may be subsequently removed under neutral, aqueous conditions using water soluble phosphines or thiols allowing further extension”
137. At paragraphs [0020]-[0024] further details are given about the nucleotide itself and the idea of using a linker to link the base to a detectable label. The idea of incorporating the detectable label into the blocking group instead is also mentioned (paragraph [0023]). Further details of suitable labels and linkers are given in a passage from paragraph [0063] to [0090]. The preferred detectable labels are fluorophores, which can be detected by fluorescence. The linker may be a ‘cleavable linker’ which is described at paragraph [0068] as being one which ensures that the label can, if required, be removed after detection, avoiding any interfering signal with any labelled nucleotide incorporated subsequently. At paragraph [0077] the specification explains that a ‘cleavable linker’ does not require the whole linker to be removed. Part of it can remain attached to the base. Examples of suitable linkers and their method of cleavage are given.
138. At paragraphs [0104] to [0139] the synthesis of modified nucleotides with a 3' azidomethyl blocking group, linked to a fluorescent dye via a linker moiety, is described. Syntheses of modified versions of all four of G, C, A and T, with the triphosphate and a 3' azidomethyl group are provided. Compound 6, 18, 24, and 32 are used in experiments. They are dTTP, dCTP, dGTP and dATP respectively.
139. Experiments showing more than one cycle of incorporation, blocking and de-blocking using the modified nucleotides are described with the results shown in the gels of Figs. 5 and 6. The experiments used radiolabelled hairpin primers, attached to beads, into which the modified nucleotides were incorporated. There were three stages: (a) incorporation of the modified nucleotide; (b) a chase by native unmodified nucleotides to check that incorporation of the modified nucleotide and thereby blocking of further incorporation had occurred; and (c) deblocking of the modified nucleotide to remove the blocking group and fluorescent label. This is depicted in Prof Leadlay’s diagram:

140. At each stage, beads were removed from the reaction and the DNA was released from the beads onto a gel to allow analysis of the reaction products. The position of the bands on the gel corresponds to the size of the DNA: larger molecules move more slowly and are therefore visualised higher up the gel than smaller ones. The radiolabel on the hairpin permits the bands to be visualised.
141. Fig. 5 shows the results for compounds 24, 18 and 32 (i.e. G, C and A). Prof Leadlay provided an annotated version of the figure (below). I agree the skilled reader would see it that way. I was not persuaded by a piece of evidence from Prof Marx that the absence of labelling in the patent meant that any interpretation had to be based on a common general knowledge expectation that azidomethyl would be incorporated.
142. The annotated Fig. 5 is:
143. The ‘hairpin’ band (to the left of each set) shows the position of the hairpin primer (prior to any incorporation) on the gel. Moving from left to right, the bands show the position of the DNA following the first cycle of incorporation; chase and deblocking phases and then the second cycle. The higher position of the band indicates a larger DNA molecule and hence shows that incorporation has occurred. Similarly, the lower position of the band indicates a smaller DNA molecule, and hence shows that de-blocking (and removal of the fluorescent label) has occurred.
144. The first cycle for compounds 18 and 32 show complete incorporation and de-blocking of the modified nucleotides. The first cycle for compound 24 shows that there was also incorporation and de-blocking, however it was not complete as there is a faint band at the same position as the hairpin primer band in lane 1, indicating that some hairpin primers remained into which no nucleotide had been incorporated.
145. Fig. 6
shows the results for compound 6 (T) which was the subject of six cycles of incorporation, chase and deblocking. Again Prof Leadlay provided an annotated version which I accept:
146. As with Fig. 5, the lanes from left to right represent: hairpin primer followed by the first and then subsequent rounds of incorporation, chase and de-blocking. The results for the first three cycles show clearly that a modified nucleotide incorporates into the polynucleotide at each cycle and is then successfully de-blocked.
147. In general in both sets of gels, the bands widen as one goes from left to right. Little can be made out relating to the second cycles in Fig. 5 and by the fourth cycle in Fig. 6. Prof. Leadlay puts this down to overexposure of the audio-radiograph in these lanes which was caused by uneven loading of the products into the gel between cycles. Prof Marx did not agree. His view was that the two cycle reactions for G, C and A and the six cycle reaction for T were not fully efficient and the smears suggested the presence of unwanted side products.
148. At paragraph [0147] the specification states in terms that two cycles of incorporation with compounds 18, 24 and 32 and six cycles with compound 6 are seen in the figures. I am sure the skilled person would think overexposure and loading was part of the explanation for what is seen but I was not convinced by Prof Leadlay that the skilled person would see that as the whole explanation. Bearing in mind Prof Marx’s evidence I find that the skilled person would not simply disbelieve or reject what is said in paragraph [0147]. Rather they would accept that the second cycle for C, and A (less so for G) and the fourth and later cycles for T were likely to have taken place but may not have been fully efficient, and may have involved side reactions.
149. However while I have accepted part of Prof Marx’s evidence here at a technical level, I was not persuaded by his view that the contents of the patent did not did not represent an important or significant development. To characterise the patent as MGI sometimes did in argument as just showing one more cycle (three) compared to two cycles shown in the prior priority date papers is not realistic and not how it would be viewed by the skilled person.
150. Counsel for Illumina put to Prof Marx that this experimental data was a significant technical advance in the field of reversible chain termination sequencing. Prof Marx did not accept that because of the quality of the data as summarised above. He was prepared to accept that the third cycle of incorporation of the T nucleotide was a step which had not been shown before but he would not accept it was an important or significant development. This was the least persuasive part of Prof Marx’s testimony and on this topic I preferred the evidence of Prof Leadlay. Prof Leadlay’s view was as follows. The data shows that modified nucleotides with a 3' azidomethyl blocking group may be used for controlled, one at a time, incorporation of nucleotides into a polynucleotide. The 3' azidomethyl modified nucleotides were incorporated by the polymerase, resulting in chain termination. The blocking group and fluorescent label are capable of being removed using a water-soluble phosphine to regenerate the 3' hydroxyl, allowing further rounds of incorporation of 3' blocked nucleotides in a stepwise manner. I accept this evidence.
151. In terms of efficiency, based on Prof Leadlay’s evidence, I find that the skilled person taking the patent as a whole including these figures 5 and 6 would conclude that while further optimisation of the conditions was likely to be required, they would expect it to amount to routine work. They would regard the data as showing that the incorporation and deblocking steps were sufficiently efficient to be a promising repeatable technique. The worst one was for compound 24 (G) but it was not so bad that the skilled person would think it would not work. Furthermore (and this shades into the insufficiency arguments but it is convenient to mention this now) based on Prof Leadlay’s evidence I find that it would in fact be routine work for the skilled person to carry out.
152. Overall, the skilled person reading the patent as a whole and taking into account the experimental results, would accept as plausible the proposition that a nucleotide with a 3' azidomethyl blocking group satisfied the objectives set out by paragraphs [0004] and [0005].
Claim construction
153. The law on the construction of patent claims is well established and there is no need to set it out. I will deal with infringement below, after validity.
154. Annexed below are three sets of claims which relate to the three modified nucleotide patents. Claim set A relates to the  578
578 patent. Claim set B relates to 289 and claim set C relates to 433.
patent. Claim set B relates to 289 and claim set C relates to 433.
The claims of the  578
578 patent
patent
155. Claim set A consists of the claims of  578
578 as proposed to be amended as at the end of the trial. All the changes are unopposed and unconditional except one. The one which is conditional is the amendment to claim 12 as granted (claim 7 in claim set A). It would cure the alleged lack of technical contribution. Claim set A is shown in red and green because it consists of two successive sets of amendments (red first and then green). Much of the claim numbering referred to at trial used the numbering which is neither as granted nor as now amended.
as proposed to be amended as at the end of the trial. All the changes are unopposed and unconditional except one. The one which is conditional is the amendment to claim 12 as granted (claim 7 in claim set A). It would cure the alleged lack of technical contribution. Claim set A is shown in red and green because it consists of two successive sets of amendments (red first and then green). Much of the claim numbering referred to at trial used the numbering which is neither as granted nor as now amended.
156. Whereas claim 1 of  578
578 as granted was to a modified nucleotide with a range of O linked blocking groups at the 3' position, as proposed to be amended claim 1 is limited to azidomethyl. This change also involves removing granted claims 2 to 5 (claim 4 to azidomethyl has been collapsed into new claim 1). The claims have been renumbered accordingly.
as granted was to a modified nucleotide with a range of O linked blocking groups at the 3' position, as proposed to be amended claim 1 is limited to azidomethyl. This change also involves removing granted claims 2 to 5 (claim 4 to azidomethyl has been collapsed into new claim 1). The claims have been renumbered accordingly.
157. Claim 1 calls for a modified nucleotide molecule comprising a base and sugar. The base can be a purine or pyrimidine. The sugar can be ribose or deoxyribose. No points of construction arise on these expressions in the claim. The molecule has to have a removable 3'-OH blocking group covalently attached to it, and (as amended) the blocking group attached to the 3' carbon is O-azidomethyl.
158. Claim 2 of claim set A (claim 6 as granted) calls for a molecule of claim 1 in which the base is linked to a detectable label. The linkage is via a cleavable linker or a non-cleavable linker. There is an issue about the construction of this claim which arises because claim 12 (claim set A) is alleged to be infringed by Cool MPS and that claim is dependent on this claim 2 (claim set A). The issues are whether the link must be covalent and to what must the link be connected. These are best addressed in the infringement section below.
159. MGI also draw attention to the point that in the form as granted this claim (claim 2 claim set A) had a counterpart in claim 8 as granted. Whereas what is now claim 2 (claim set A) relates to the detectable label being attached by the linker to the base, the counterpart granted claim 8 required the detectable label to be attached by the linker to the blocking group.
160. Claim 3 (claim set A) is limited to cleavable linkers, excluding the non-cleavable option in claim 2.
161. Claim 7 of claim set A (claim 12 as granted) relates to a method of controlling the incorporation of a complementary nucleotide in a reaction using a single stranded target. The nucleotide to be incorporated is a modified nucleotide as defined in claims 2 to 5 (claim set A). With this claim dependency the modified nucleotide is limited to one in which the detectable label is connected to the base by a cleavable (or non-cleavable) linker. The claim refers to incorporating the nucleotide into the growing complementary strand and also requires that the incorporation prevents or blocks introduction of a subsequent nucleoside or nucleotide.
162. As granted the reaction defined in this claim can be a synthesis or a sequencing reaction. The alleged lack of technical contribution relates to the option of its being a synthesis reaction. That challenge does not apply to the claim if it is limited to a sequencing reaction.
163. Claim 12 of claim set A (claim 17 as granted) is to a method of determining the sequence of a target single stranded polynucleotide. The method involves monitoring the sequential incorporation of complementary nucleotides and requires that “at least one” incorporation is of a nucleotide claimed in claims 2 to 5 (claim set A). In other words as with the claim to a method of controlling incorporation, by the claim dependency this nucleotide has to be a modified nucleotide with the azidomethyl of claim 1 but also with a label linked to the base by a linker (claim 2). The method also involves determining the identity of the nucleotide by detecting the label linked to the base. Finally it requires the blocking group and the label to be removed prior to the introduction of the next complementary nucleotide.
164. The fact the claim relates to “at least one” incorporation gives rise to an issue which is best addressed in the Regeneron insufficiency section below.
165. Claim 20 of claim set A (claim 25 as granted) is a claim to a kit. The kit comprises packaging material and also a plurality of different nucleotides. The claim provides that the nucleotides are as defined in claims 2 to 5. So again by the claim dependency this relates to azidomethyl blocked nucleotides with a detectable label linked to the base by a linker. There is an issue about the scope of this claim - does it cover a system in which some of the nucleotides are modified (as required by claims 2 to 5) and some are not? This is best dealt with along with infringement.
166. Claim 24 of claim set A (claim 29 as granted) relates to an oligonucleotide comprising a modified nucleotide of claims 1 to 6 (of claim set A). No issue of construction arises.
The claims of the 289 patent
167. Claim set B consists of the claims of 289 as proposed to be amended as at the end of the trial. All the changes are unopposed and unconditional.
168. Claim 1 is to a modified nucleotide triphosphate molecule with a 3' azidomethyl group. It comprises a purine or pyrimidine base and a deoxyribose sugar moiety. No issue of construction arises in relation to it.
169. Claim 2 is to the molecule of claim 1 with a detectable label linked to the base by a linker. The option that the linker was non-cleavable is deleted by the amendment and so claim 2 as amended is limited to using a cleavable linker. Accordingly claim 3 as granted is removed.
170. Another claim deleted by the amendment is what was granted claim 5 (to the linker containing a phosphine cleavable azide). Nothing turns on that in this case.
171. Claim 4 of claim set B (claim 6 as granted), is a claim to a kit. It is worded in a different way to the kit claim of  578
578 (claim 20 of claim set A) but a similar infringement issue arises as it does for claim 20 of
(claim 20 of claim set A) but a similar infringement issue arises as it does for claim 20 of  578.
578. The four modified nucleotides in the kit each comprise a purine or pyrimidine base, a deoxyribose sugar moiety and a 3' azidomethyl group. They also have a detectable label linked to the base by a cleavable linker. There is also a point on infringement of this claim by Cool MPS related to “cleavable linker” which is best dealt with in context.
The four modified nucleotides in the kit each comprise a purine or pyrimidine base, a deoxyribose sugar moiety and a 3' azidomethyl group. They also have a detectable label linked to the base by a cleavable linker. There is also a point on infringement of this claim by Cool MPS related to “cleavable linker” which is best dealt with in context.
172. Claim 5 of claim set B (claim 9 as granted) is to a polynucleotide. It is independent of claim 1. As amended the polynucleotide comprises a modified nucleotide with a purine or pyrimidine base, a deoxyribose sugar moiety and a 3' O-azidomethyl group. Infringement of this claim by Cool MPS is admitted. No issues of construction arise on it.
173. Claim 6 of claim set B (claim 10 as granted) is a claim to a method for determining the sequence of a target single-stranded nucleotide. The same “cleavable linker” point arises on this claim in relation to Cool MPS, and is best dealt with in context.
174. The other notable claim of 289 is claim 9 of claim set B (claim 13 as granted). This claim defines part of the method claimed in earlier claims such as claim 6 by reference to removing the blocking group using a water soluble phosphine. By amendment Illumina seek to add the words “under neutral, aqueous conditions”. MGI contends this amendment adds matter.
The claims of the 433 patent
175. Claim set C consists of the claims of 433 as proposed to be amended as at the end of the trial. The claim numbering is the same in Claim set C and as granted.
176. Claim 1 of 433 is to a kit comprising four modified nucleotides, each having a purine or pyrimidine base, a deoxyribose sugar moiety and a 3' O-azidomethyl group. No points of construction arise on this claim.
177. Claim 6 of 433 relates to a method of incorporation similar to the corresponding claim of  578.
578. The corresponding claim is claim 7 of claim set A (claim 12 of
The corresponding claim is claim 7 of claim set A (claim 12 of  578
578 as granted). As with that
as granted). As with that  578
578 patent claim, the proposed amendment to claim 6 of 433 is to remove the reference to synthesis and so leave the claim limited to a sequencing reaction.
patent claim, the proposed amendment to claim 6 of 433 is to remove the reference to synthesis and so leave the claim limited to a sequencing reaction.
178. An important difference between claim 6 of 433 and the corresponding claim of  578
578 is that the latter is limited to a modified nucleotide in which the detectable label is linked to the base by a linker, whereas claim 6 of 433 contains no such limitation. Thus although MGI deny infringement of claim 7 of claim set A of
is that the latter is limited to a modified nucleotide in which the detectable label is linked to the base by a linker, whereas claim 6 of 433 contains no such limitation. Thus although MGI deny infringement of claim 7 of claim set A of  578
578 by Cool MPS because it uses antibody detection, MGI admits infringement of claim 6 of 433 because the nucleotide which is to be incorporated is only defined by reference to its having the right base, sugar and 3' azidomethyl blocking group.
by Cool MPS because it uses antibody detection, MGI admits infringement of claim 6 of 433 because the nucleotide which is to be incorporated is only defined by reference to its having the right base, sugar and 3' azidomethyl blocking group.
Obviousness
179. Section 1 of the 1977 Act defines a patentable invention as requiring an inventive step. Section 3 of the 1977 Act provides that “an invention shall be taken to involve an inventive step if it is not obvious to a person skilled in art, having regard to any matter which forms part of the state of the art by virtue of section 2(2) above (and disregarding section 2(3) above).”
180. The approach the court should take to testing the question of obviousness is well settled, based on the questions posed by Oliver LJ in Windsurfing v Tabur Marine [1985] RPC 59 at pp71 - 74 and reviewed by the Court of Appeal in Pozzoli v BDMO [2007] FSR 37 at paras 14 - 23, as follows:
(1) Identify:
(a) the notional person skilled in the art; and
(b) the relevant common general knowledge;
(2) Identify the inventive concept of the claim in question or if that cannot readily be done, construe it;
(3) Identify what, if any, differences exist between the matter cited as forming part of the state of the art and the inventive concept of the claim or the claim as construed;
(4) Viewed without any knowledge of the alleged invention as claimed, do those differences constitute steps which would have been obvious to the person skilled in the art or do they require any degree of invention?
181. The parties also highlighted a number of other passages from the authorities on obviousness. The passages from Lord Hodge’s judgment in the Supreme Court in Actavis v ICOS, referring as they do to passages from Kitchin J in Generics v Lundbeck and Laddie J in Brugger v Medicaid are well known and do not need to be referred to again.
182. A point made by Laddie J in Inhale v Quadrant [2002] RPC 21, which has come up more recently in other cases too, bears a reference. In paragraph 47 the judge was addressing the fiction that a skilled person is deemed to read the cited prior art with interest even if in practice they never would. He said:
“… The notional skilled person is assumed to have read and understood the contents of the prior art. However that does not mean that all prior art will be considered equally interesting. The notional skilled person is assumed to be interested in the field of technology covered by the patent in suit, but he is not assumed to know or suspect in advance of reading it that any particular piece of prior art has the answer to a problem he faces or is relevant to it. He comes to the prior art without any preconceptions and, in particular, without any expectation that it offers him a solution to any problem he has in mind. Some pieces of prior art will be much more interesting than others. … ”
183. The skilled person and common general knowledge have been identified above. Claim 1 of  578
578 (claim set A) is essentially to a modified nucleotide with an azidomethyl group on the 3' oxygen. There is no need separately to identify an inventive concept. In order to identify the differences the next task is to address the disclosure of the cited prior art.
(claim set A) is essentially to a modified nucleotide with an azidomethyl group on the 3' oxygen. There is no need separately to identify an inventive concept. In order to identify the differences the next task is to address the disclosure of the cited prior art.
Zavgorodny 1991
184. Zavgorodny 1991 is a paper about chemical synthesis. It describes a method for synthesising certain substituted nucleosides. A nucleoside differs from a nucleotide in that it lacks the 5' phosphate groups. In other words a nucleoside is just the base and the ribose (or deoxyribose). Nucleosides had a number of applications at all material times including as antiviral and anticancer agents.
185. Zavgorodny’s syntheses are summarised in the single figure in the paper, which is shown below. The synthetic scheme involves starting with a 5' blocked nucleoside and generating a 3'-O-methylthiomethyl nucleoside. That product is compound 1 in the scheme below (second from the left at the top). The term “thio” refers to the presence of the sulphur between the two methyl groups on the 3' oxygen in compound 1. The group blocking that would otherwise have been the 5' hydroxyl is a benzyl group (“Bz”). Zavgorodny uses the term alkylthioalkyl as a generalisation of the specific methylthiomethyl group used in the experiments.
186. The Zavgorodny figure is:
187. From the 3'–O-methylthiomethyl compound 1 various further routes are taken to generate a selection of O-substituted nucleosides at the 2', 3' and 5' positions. One of the suggestions is to block the 3' hydroxyl with an azidomethyl group. That is depicted in Zavgorodny’s figure by looking at compound 5 (in which the 5' end is no longer blocked) and taking “X” to be N3. X = N3 is one of the listed substituents in the figure. Compound 5 is a deoxyribonucleoside. Compounds based on ribonucleosides are also mentioned.
188. The last paragraph of Zavgorodny is as follows:
“The compounds discussed above are useful specifically blocked synthons. For example, alkylthioalkyl groups can be removed with methyl iodide, mercury(II) and silver(I) salts, tritylium tetrafluoroborate, or bromine/water treatment. O-Methoxymethyl substituted nucleosides may be deblocked according to Nishino, and acetoxymethyl and 2-cyanoethoxymethyl groups undergo elimination under alkaline conditions. Azidomethyl group is of special interest, since it can be removed under very specific and mild conditions, viz. with triphenylphosphine in aqueous pyridine at 20 ºC.”
189. The first sentence reflects the focus of Zavgorodny as a paper about synthetic chemistry. A “synthon” is a unit to be used in further syntheses. This sentence emphasises that what Zavgorodny is providing are chemical structures which, from Zavgorodny’s point of view, are themselves going to be used to synthesise other things. In other words, as Prof Leadlay said, they are synthetic intermediates. The skilled person reading this in 2002 would understand this.
190. The second sentence relates to other groups and is not important. In the context of this case the third sentence is significant. It draws attention to the azidomethyl group, saying it is of special interest and can be removed under very specific and mild conditions. However it is important to appreciate that the reference to “special interest” is not an assertion about sequencing by synthesis reactions. Zavgorodny is not suggesting that this group is of “special interest” in that context. The skilled person would understand that what Zavgorodny is talking about is in the context of its use as a synthon, a chemical intermediate. Protecting groups are a routine tool in synthetic chemistry. It would be an exercise of hindsight to read that as a disclosure concerned with sequencing by synthesis or reversible chain terminators. Whether it is obvious to employ this group as a reversible chain terminator is a different issue. At this stage I am concerned with that Zavgorodny actually discloses.
191. The same goes for the reference to removal under “very specific and mild conditions”. What Zavgorodny is referring to is specificity and mildness in the context of the work he is reporting and contemplating, i.e. organic synthesis. So, for example, “mild conditions” is not a reference to their being mild relative to the stability of DNA. Whether the conditions happen to be mild vis a vis DNA is a different question. So also the specificity referred to relates to the other groups on the molecules Zavgorodny is describing.
192. Finally a point arises on what “triphenylphosphine in aqueous pyridine at 20 ºC” means. Again read without hindsight the answer is clear enough. It refers to using triphenylphosphine in what a skilled person would regard as an organic solvent: aqueous pyridine. It is common ground that pyridine denatures DNA.
Differences over Zavgorodny
193. In a way the difference between Zavgorodny 1991 and claim 1 of  578
578 is quite small. Claim 1 claims a nucleotide (i.e. with the 5' phosphates) with an azidomethyl group at the 3' oxygen whereas Zavgorodny 1991 discloses a nucleoside with such a group at the 3' oxygen. However in order to render claim 1 invalid the skilled person has to make the claimed molecule and for that to happen the skilled person has to have a reason to do so. As a result the inventive step(s) of all the relevant claims of the modified nucleotide patents stand or fall together. If performing sequencing by synthesis using a nucleotide with an azidomethyl blocked 3' oxygen as a reversible chain terminator is obvious over Zavgorodny 1991, then claim 1 also lacks inventive step because it would be obvious to make the relevant compound. If that exercise was not obvious then none of the claims, including claim 1 of
is quite small. Claim 1 claims a nucleotide (i.e. with the 5' phosphates) with an azidomethyl group at the 3' oxygen whereas Zavgorodny 1991 discloses a nucleoside with such a group at the 3' oxygen. However in order to render claim 1 invalid the skilled person has to make the claimed molecule and for that to happen the skilled person has to have a reason to do so. As a result the inventive step(s) of all the relevant claims of the modified nucleotide patents stand or fall together. If performing sequencing by synthesis using a nucleotide with an azidomethyl blocked 3' oxygen as a reversible chain terminator is obvious over Zavgorodny 1991, then claim 1 also lacks inventive step because it would be obvious to make the relevant compound. If that exercise was not obvious then none of the claims, including claim 1 of  578,
578, are obvious for the converse reason. Another way of approaching the same question would be to ask whether it was obvious to the skilled person given Zavgorodny 1991, with a reasonable prospect of success, to try out a sequencing by synthesis test using a nucleotide with an azidomethyl blocked 3' oxygen as a reversible chain terminator.
are obvious for the converse reason. Another way of approaching the same question would be to ask whether it was obvious to the skilled person given Zavgorodny 1991, with a reasonable prospect of success, to try out a sequencing by synthesis test using a nucleotide with an azidomethyl blocked 3' oxygen as a reversible chain terminator.
194. At one stage MGI had an alternative case that the molecule of claim 1 was obvious over Zavgorodny irrespective of sequencing by synthesis because it would be obvious as a candidate antiviral. That case was dropped before trial.
Is it obvious?
195. As I have already mentioned, Prof Marx’s evidence that the invention was obvious was based on a premise that the skilled person would look at Zavgorodny with the specific aim in mind of finding a blocking group they might be able to use in a reversible chain terminator sequencing process, and the cross-examination of Prof Leadlay was on the same premise. However I have rejected this premise. It is tempting therefore simply to stop at this point and find the claim is not obvious.
196. Prof Winssinger’s opinions were also founded on a premise which I have found does not reflect the thinking of the skilled person. In his case it was the relevance of the Bertozzi work on azides in biomolecules. Despite the fact that the existence of that work was part of the common general knowledge, it would be irrelevant. The skilled person had no reason to think about it in the context of their knowledge of sequencing by synthesis and I reject the idea that reading Zavgorodny would cause them to reflect on the Bertozzi work at all. An imaginative skilled person might note that it was a reference to an azide, just as Bertozzi was about azides too, but that is the limit of it. No useful connection would be made. I reject the idea that a skilled person knowing of Bertozzi would read Zavgorodny in 2002 in a different way from a skilled person ignorant of Bertozzi.
197. However this is still not the end of the matter. The purpose of expert evidence in a patent case is to educate the court, to express opinions on the issues and crucially to give reasons for those opinions. Even if the conclusions expressed by an expert on obviousness were on a premise which the court has rejected, it is still necessary for the court to look at the evidence as a whole and come to a conclusion.
198. The skilled person is a team working on research into sequencing by synthesis. They are aware of the idea of using reversible chain terminators but as far as they were concerned the idea had not succeeded. They knew that to make it work they would need to come up with a system in which one could repeatably incorporate a nucleotide linked to a specific label, one at a time, in a reversible way. They did not have any specific problem or problems in mind which had to be solved as a key to unlock the ability to take the method forward. It may well have been that the technique simply could not be made to work.
199. As a matter of principle, as with any item of prior art, the skilled person is deemed to read Zavgorodny with interest. They would see that it was a paper concerned with chemical intermediates (synthons). They would see that one such intermediate was a nucleoside in which the 3' OH had been blocked with azidomethyl. They would see the reference to removal using mild and specific conditions and that Zavgorodny regarded the azidomethyl group as of special interest. In my judgment the most likely thing such a skilled team would think having read Zavgorodny is that this paper on synthetic chemistry had nothing to do with their focus on sequencing by synthesis. To the skilled person the concept of protecting groups in synthetic chemistry is commonplace. At most Zavgorodny would be seen as something to add to the organic chemist team member’s general toolbox concerning chemical synthesis (see Prof Marx in cross-examination at T6/729). There is simply nothing, absent hindsight, to suggest that what is disclosed here has an application in relation to sequencing by synthesis using reversible chain terminators. They would read it with interest and having done so, put it down and move on.
200. One thing which is not important is the point that Zavgorodny specifically describes a nucleoside rather than a nucleotide with its triphosphate. That is not a reason why the invention is not obvious.
201. Even if the skilled person saw an analogy between the blocking 3' end in Zavgorodny and the idea of blocking the 3' end of a nucleotide in sequencing by synthesis, it would not make the invention obvious. The skilled person did not think they needed a new group to try as a reversible chain terminator. The skilled person knew that there were numerous possible candidate groups and would be aware that there was a textbook in which to find such things if they had wanted help with thinking of some to try (Greene & Wuts).
202. The conditions needed to remove the blocking group obviously matter in the abstract, but the reference to removal under specific and mild conditions here does not assist MGI very much. If the skilled person had thought that it was the removal conditions which were a particular problem and were the reason why sequencing by synthesis using reversible chain terminators had stalled as a concept, then it might be different, but that is not the case.
203. The skilled person would understand the reference to specific and mild conditions as a reference to the circumstances of Zavgorodny itself rather than a suggestion about the properties of this group in conditions required for DNA synthesis. On the other hand as a matter of the common general knowledge of the organic chemist member of the team, if they did get this far, they would think that they would be able to select conditions using their own skill which would be likely to remove such an azide group from a nucleotide without being likely to cause difficulties for DNA. That is why the “pyridine point” raised by Illumina is a bad point. It is true that the skilled person would be unlikely to want to use the particular removal conditions referred to in Zavgorodny (triphenyl phosphine in aqueous pyridine). That is because pyridine is known to denature DNA. But the skilled person would be well aware of that and as I have said, would be aware of suitable aqueous conditions using phosphines which would be expected to remove an azide without being such as to denature DNA.
204. Even if the skilled person got as far as considering whether to try out an azidomethyl group as a 3' blocking group on a nucleotide in a test of a single cycle, they would, as Prof Leadlay explained, have no basis for thinking that such a blocked nucleotide would be incorporated into an oligonucleotide by DNA polymerase. It might or it might not.
205. I reject the submission of MGI’s that because an azidomethyl group is small, that would support a prospect of successful incorporation. On the contrary, the evidence as at 2002 does not allow that conclusion to be drawn. Azidomethyl is larger than a number of the groups tested in Metzker some of which failed completely (O-acyl), and others of which only worked inconsistently (O-methyl - which worked with the A base only using reverse transcriptase and with the T base only with some DNA polymerases). I accept Prof Leadlay’s summary of the relevance of size which he gave in cross-examination: too big is bad but small is not necessarily beautiful.
206. Another submission was based on an analogy with the successful drug AZT used to treat HIV. This is a nucleoside in which an azide group replaces the OH at the 3' position and blocks the chain. Unlike the invention, there is no methyl group nor a 3' oxygen in AZT.
207. In order to interfere with HIV infections, the idea is that the AZT molecule is incorporated into a growing DNA chain by the reverse transcriptase enzyme of HIV but then blocks further DNA synthesis, thus stopping HIV from successfully infecting someone. Whereas it is not picked up by human DNA polymerase (otherwise it might harm the host). There was a point on whether it was picked up by bacterial DNA polymerases but, in case it matters, I find the published work on that was not common general knowledge.
208. The real points on AZT however are first that the skilled person would not think of it as having any relevance if they were considering the prospects of trying out a 3' azidomethyl blocking group as a reversible chain terminator. Furthermore, even if they did, thinking of AZT does not provide a basis for a reasonable prospect of success because the differences in chemical structure mean it cannot be assumed to behave in the same way.
209. A final dimension to the question of removal conditions is the following. While the skilled person would think they could come up with conditions in which to remove an azidomethyl group without damaging DNA, that is not the only issue. To be useful in sequencing by synthesis the removal has to have a reasonable yield and reasonable speed.
210. In my judgment the position is simply that the skilled person has no basis from which to infer that there was a reasonable prospect of getting a reasonable yield and speed.
211. Illumina sought to go further and suggest that the true position was positively against reasonable yield and speed. This was in Prof Leadlay’s evidence on the basis that the skilled person thinking of O-azidomethyl as a blocking group would look it up in Greene & Wuts and find a 1988 paper by Loubinoux which reported only unpromising 60%-80% yields and long reaction times. Prof Leadlay was not challenged on this but I was not persuaded the skilled person would undertake such a paper chase.
212. MGI also sought to go further in the opposite way by seeking to establish a positive case that the expectation would be of reasonable yields and speed. This was by reference to the work of Bertozzi. However again I was not persuaded that the skilled person would think any useful analogy could be drawn between that work and an attempt to use an azidomethyl blocking group in sequencing by synthesis. However even if it was, I was also not convinced the exercise produces a clear result in MGI’s favour. Looking at the Bertozzi work as a whole, as the skilled person would, if they got that far, see that there is evidence of a need for long times (6 hours) to produce optimal yield.
ICOS factors
213. MGI contended that the factors summarised in ICOS all point in favour of a finding of obviousness. The points are:
i) Obvious to try - I reject MGI’s submission that there was a sufficient likelihood of success to warrant trying incorporation of the azidomethyl with a range of standard polymerases. There would be no such expectation.
ii) Routine work - The work actually involved in testing a range of polymerases or testing deprotection is not difficult to do.
iii) Cost - Cost is not a relevant factor in this case.
iv) Value judgments - This is not a case about multiple value judgments. However deciding to try out azidomethyl in a sequencing by synthesis test does not follow from Zavgorodny.
v) Multiple paths of research - does not apply here.
vi) Motive - I reject MGI’s submission that this points in favour of obviousness. On the contrary it points against for the reasons already addressed.
vii) Unexpected result - A 3'-O-azidomethyl blocking group has the useful features promised by the patent in paragraph [0004 and [0005]. That was not predictable from the prior art.
viii) Step by step analysis - This is not a major factor in the present case.
ix) Added benefit - This is not a bonus effect case.
The deposition of Dr Liu
214. Under a Civil Evidence Act notice MGI relied on a passage in a deposition of Dr Xiaohai Liu of Solexa in USA proceedings between Illumina and the Trustees of Columbia University. Dr Liu is one of the named inventors on the modified nucleotide patents. The deposition was in 2013. In the deposition Dr Liu was shown Zavgorodny 1991. The testimony MGI relies on is an answer Dr Liu gave when he was shown the passage about azidomethyl being of special interest which is quoted above. The questioner put to him that that passage would suggest that an azidomethyl group might be something to try as a protecting group for SBS. His answer was: “That’s a perfectly valid argument. Yeah, I agree with you. You probably thinking about it; use it, yes.”
215. I will not place any weight on this deposition for the following reasons. Dr Liu is one of the inventors. It is unlikely that he represents the notional skilled person armed only with the common general knowledge. For example the fact that the inventors may have decided that finding new blocking groups was important from their point of view does not mean that that was the attitude of the uninventive skilled person. Since no attempt was made before me to establish that Dr Liu was approaching the answers he gave in the legally relevant way, this view has no bearing on the questions I have to decide.
Inventive step - conclusion
216. Standing back, for the reasons explained above claim 1 of  578
578 is not obvious over Zavgorodny 1991. I reject MGI’s case on lack of inventive step. As mentioned at the outset, if the claim is not obvious over Zavgorodny 1991 then it is not obvious over Zavgorodny 2000 either.
is not obvious over Zavgorodny 1991. I reject MGI’s case on lack of inventive step. As mentioned at the outset, if the claim is not obvious over Zavgorodny 1991 then it is not obvious over Zavgorodny 2000 either.
217. If claim 1 is not obvious then neither is claim 12 of claim set A (to a sequencing method) nor claim 24 of claim set A (an oligonucleotide comprising a modified nucleotide of claim 1).
Secondary evidence
218. Illumina relied on secondary evidence at least to some extent, submitting as follows. Azide chemistry was not unknown, azides were even known as blocking groups and azidomethyl was not an unknown chemical group. There were a number of disparate instances of papers in which protecting groups for reversible chain terminator sequencing have been suggested (including Tsien, Ju, some of the patent applications in 2000-2002, Metzker and Canard). Nevertheless none of them even suggest azidomethyl and Illumina pointed out that Prof Marx accepted he could not explain why not.
219. Illumina also referred to post published material in the form of a 2005 paper from the Ju group by Ruparel and a 2004 paper from Prof Church’s group by Shendure. The Ruparel paper (passage quoted below) supports the idea that in fact finding as successful reversible blocking group for the 3' end was a formidable challenge (as Prof Marx accepted) and it seems that the Ju group adopted the azidomethyl approach after learning of its use by the inventors. In relation to other blocking groups Ruparel states:
“Significant efforts have been dedicated for evaluating a wide variety of 3’ modified nucleotides to be used as terminators for various DNA polymerases and reverse transcriptases, but none of the functional groups tested have had established methods to regenerate a free 3-OH.”
220. The Shendure paper states that “developing reversible terminators with the necessary properties has proved to be a difficult problem”.
221. However I have preferred to approach this case without taking this secondary evidence into account. That is because neither Zavgorodny paper was shown to be known by those working on sequencing by synthesis at any relevant time. One could get into an argument about whether Zavgorodny adds to the common general knowledge since azides were well known, but that was not argued and I will not address it.
222. Finally, I should note that I do not accept that those post-published papers indicate that it was understood before the priority date that the problem with sequencing by synthesis using reversible chain terminators would be solved by finding a suitable reversible blocking group. That is hindsight.
Obviousness - lack of technical contribution
223. The claims to a method of controlling incorporation, which are claim 7 (claim set A) of the  578
578 patent and claim 6 (as granted) of the 433 patent, are both defined in such a way that the method is defined as applicable in a synthesis or a sequencing reaction. MGI contended that insofar as the claims covered the method in “a synthesis reaction not being a sequencing by synthesis reaction”, then the claim had no technical benefit over the common general knowledge or prior art and so the claims are invalid for lack of inventive step on the Agrevo basis. The same argument was put under the heading of insufficiency. Illumina denied the invalidity but offered an amendment to delete synthesis, which would cure it. This point is MGI MNP issue 3.
patent and claim 6 (as granted) of the 433 patent, are both defined in such a way that the method is defined as applicable in a synthesis or a sequencing reaction. MGI contended that insofar as the claims covered the method in “a synthesis reaction not being a sequencing by synthesis reaction”, then the claim had no technical benefit over the common general knowledge or prior art and so the claims are invalid for lack of inventive step on the Agrevo basis. The same argument was put under the heading of insufficiency. Illumina denied the invalidity but offered an amendment to delete synthesis, which would cure it. This point is MGI MNP issue 3.
224. MGI’s case, as put in paragraph 341 of its written closing, is:
“[…] One [point] relates in essence to the patentee’s failure to limit his claims to the use of azidomethyl in methods in which its reversible nature is of utility, i.e. SBS methods. Instead the patentee has claimed greedily, attempting to throw the claims wider to cover the use of azidomethyl in methods of synthesis and Sanger sequencing. In both cases the claims cover embodiments in which the use of azidomethyl simply represents an alternative chain terminator, whose selection is not justified by any useful technical property. […]”
225. MGI submitted that there were well-known methods of DNA synthesis such as PCR and phosphoramidite synthesis reactions in which while one could use an azidomethyl group, it would serve no useful purpose. Therefore the selection of an azidomethyl blocked nucleotide for use in such methods is not justified by any useful technical property and so the claim is broader than that which is justified by the patentee’s contribution to the art. MGI referred to the (unchallenged) evidence of Prof Marx that the skilled person would not think that nucleotides with 3’O-azidomethyl groups would be useful in either the PCR or phosphoramidite method. MGI contended that in Prof Leadlay’s written evidence he had said that he saw no reason why the skilled person would seek to use the claimed nucleotides in such methods. In response Illumina relied on evidence given by Prof Leadlay that there was utility in using nucleotides with 3’O-azidomethyl groups other than in a RCT sequencing by synthesis reaction. He explained it could be used in Sanger sequencing as a chain terminator.
226. I accept Prof Marx’s view that the skilled person would not think there was any utility in using those nucleotides in either the PCR or phosphoramidite method. However based on Prof Leadlay, I also find that nucleotides with 3’O-azidomethyl groups could be used as chain terminators in a Sanger sequencing reaction. However this latter point made by Prof Leadlay relates to the limb of the claim related to sequencing and does not meet the point made by MGI based on Prof Marx’s evidence, which relates to the different, synthesis, limb of the claim.
227. I find that the claim as drafted, which includes a distinct and express option covering synthesis, which is different from sequencing, serves no useful purpose and is invalid on Agrevo grounds.
228. I will therefore allow the amendment to delete synthesis from the two claims.
229. A second point on claim 6 of 433 also arises. It does not relate to claim 7 (claim set A) of  578.
578. The point is that claim 6 of EP 433 is not limited by an express requirement that the modified nucleotide had to be linked to a detectable label. MGI says that exceeds the technical contribution and leads to invalidity (Agrevo or insufficiency conditional on non-obviousness of claim 1). Note that it is the absence of that limitation which means the claim is infringed by the antibody based Cool MPS technique. This is MGI MNP Issue 4.
The point is that claim 6 of EP 433 is not limited by an express requirement that the modified nucleotide had to be linked to a detectable label. MGI says that exceeds the technical contribution and leads to invalidity (Agrevo or insufficiency conditional on non-obviousness of claim 1). Note that it is the absence of that limitation which means the claim is infringed by the antibody based Cool MPS technique. This is MGI MNP Issue 4.
230. MGI relies on the evidence of Prof Marx that there were no sequencing methods known at the priority date (2002) which used reversibly terminated nucleotides but with no detectable label attached to the nucleotide. Therefore it is said that the claim is broader than is justified by its contribution to the art.
231. I reject this as a ground of invalidity based on exceeding the technical contribution, whether it is couched as Agrevo obviousness or as insufficiency. The short answer to it is Prof Leadlay’s evidence about using an azidomethyl group in Sanger sequencing. That would be a way of carrying out a sequencing reaction, using the method of claim 6, without having a detectable label linked to the base of the modified nucleotide. The labelling could be at the 5' end of the strand instead. I find it would work. It is true that using an azidomethyl group in this way does not involve taking advantage of its reversibility, but that does not matter. It does take advantage of its blocking facility. The method would work and therefore this aspect of the claim has utility. It is enabled by the patent. The claim does not exceed the technical contribution. On that finding Regeneron (see below) is not relevant. Lest it be thought otherwise I do not accept that it would have been obvious to use an azidomethyl blocked nucleotide in Sanger sequencing at the priority date, absent the patent, but that is not the way the argument was put anyway.
232. I will also say that I have doubts that this argument was right in principle irrespective of the evidence. That is because the claim is to a method of controlling incorporation of a nucleotide in sequencing reaction. Although it is a part of a sequencing reaction, the claim is not focussed on the detection step and therefore there is nothing untoward about the fact that the claim does not refer to linkers or detectable labels at all. There is no assertion or promise that the claimed method will work in any situation defined by reference to the presence or absence of features which are not mentioned. All that is required for success is that the nucleotide which is incorporated has a 3' O-azidomethyl blocking group and that the incorporation blocks the next nucleotide. There is no suggestion that that does not work. However since the argument was not put this way and since the point fails on the facts, it is not necessary to examine this any further.
Priority
233. Priority is important because (as explained in the next paragraph) if Barnes is relevant prior art to claims 1, 12 or 24 (claim set A) of  578
578 then those claims would be invalid. It will be relevant prior art if they are not entitled to claim priority from the second priority document (filed on 12th Dec 2002).
then those claims would be invalid. It will be relevant prior art if they are not entitled to claim priority from the second priority document (filed on 12th Dec 2002).
234. Barnes discloses an azidomethyl group amongst a list of possible blocking groups to use as a reversible chain terminator at the 3' O position on a nucleotide in sequencing by synthesis. It is in figure 3 (top right) when R4 and R5 are H. Prof Marx’s evidence was that it would be obvious to follow that up. I agree that it would be. I think MGI sought to draw a parallel between the reasons why they said azidomethyl was obvious over Barnes and the obviousness case over Zavgorodny. There is no such parallel. The situations are quite different. Barnes discloses the idea of carrying out a method of sequencing by synthesis using reversible chain terminators. Figure 3 is provided as setting out some examples of suitable protecting groups for use in that very context (see p5 para [0062]). The document would be understood to be suggesting that those groups, including azidomethyl, had suitable properties for use in that very reaction system. The reader of Barnes would see it as suggesting that azidomethyl, as well as others, would be likely to be incorporated and removed in a useful manner as a reversible chain terminator for sequencing by synthesis. The fact that other groups are obvious over Barnes too does not make it inventive to choose azidomethyl as the way forward.
235. Turning to priority, the legal test was not in dispute. Illumina referred to Icescape v Ice-World [2018] EWCA Civ 2219 at paragraphs 38-42. The priority document must contain sufficient material to constitute an enabling disclosure of the claims in issue and in determining the question the Court will consider the position through the eyes of the skilled person who reads the priority document with their common general knowledge.
236. MGI’s point is that the gels which form part of the granted patent specification are not in the second priority document and, it asserts, the document contains no data which purports to be the results of an actual experiment. The argument is run as an attempt at a squeeze as compared to the obviousness case over Zavgorodny. I will come back to that at the end.
237. The first point is that Illumina submitted and I accept that there is textual support in the document for all the relevant claims (1, 12 or 24 of claim set A of  578).
578). In other words, in summary, the idea of carrying out sequencing by synthesis using 3' O-azidomethyl blocked nucleotides, in particular, as reversible chain terminators is disclosed in the second priority document. For what it is worth azidomethyl is not simply an entry in a list. Example 1 of the second priority document relates expressly to using an azidomethyl group to protect the 3' OH.
In other words, in summary, the idea of carrying out sequencing by synthesis using 3' O-azidomethyl blocked nucleotides, in particular, as reversible chain terminators is disclosed in the second priority document. For what it is worth azidomethyl is not simply an entry in a list. Example 1 of the second priority document relates expressly to using an azidomethyl group to protect the 3' OH.
238. MGI contends that the second priority document contains no data to support the claimed utility. Even in its own terms that is not the whole story. Example 1 on page 23 of the document specifically provides:
Nucleotides bearing this blocking group [O-azidomethyl] at the 3' position have been shown to be successfully incorporated by a number of different polymerases, block efficiently and may be subsequently removed under neutral, aqueous conditions using water soluble phosphines or thiols allowing further extension:

239. This assertion does not have any graphs or gels associated with it, but it is a statement that experiments have been done and that they were successful in various specific ways which are relevant to success from the point of view of the skilled person. Prof Leadlay’s evidence, which I accept, was that this document:
“clearly discloses (whereas Zavgorodny 1991 and Zavgorodny 2000 do not) the utility of 3'-O-azidomethyl blocked nucleotides as reversible chain terminators in a sequencing by synthesis method. For example, [the document] discloses on page 23 that such modified nucleotides have been shown to be successfully incorporated by a number of different polymerases, block efficiently, and may subsequently be completely removed with 100% yield under neutral, aqueous conditions using water soluble phosphines or thiols, allowing further extension of the oligonucleotide chain.”
240. Based on this, it seems to me that the conclusion that the claims are entitled to priority must follow. The same invention is disclosed in both the priority document and the patent. The disclosure in the priority document supports the claims and is an enabling disclosure. It also provides plausible information which supports the idea that a sequencing by synthesis scheme based on the claimed 3' O-azidomethyl blocking group will work.
241. One might have a degree of scepticism, from the way example 1 is written, the assertion of 100% yield, and the absence of gels, whether such a test really had been carried out or whether this was a so called “prophetic” example. However on the facts of this case I find that that does not matter. It does not render what is described any less plausible.
242. There is no squeeze relating to Zavgorodny and no inconsistency between this conclusion and the finding of non-obviousness. That deals with MGI MNP Issues 6 and 7.
Insufficiency
243. MGI contended that if, contrary to their primary case, claims 7 and 12 of the  578
578 patent (claim set A) are not obvious over Zavgorodny then those claims are insufficient. There are two issues.
patent (claim set A) are not obvious over Zavgorodny then those claims are insufficient. There are two issues.
244. The first one relates to read length. The point is that claim 12 (for example) claims a method for determining the sequence of a target single stranded nucleotide wherein at least one incorporation (my emphasis) is of a nucleotide defined in previous claims such as claim 2, in other words an azidomethyl 3' blocked nucleotide in which the base is linked to a detectable label by a linker. MGI says that this claim is open ended in terms of the length of the nucleotide to be sequenced and submits that the data in the patent only present results for a limited number of cycles, as shown in figures 5 and 6. Therefore, it is said, the monopoly claimed exceeds the technical contribution and the specification does not enable the skilled person to perform a sequencing method across the breadth of the claim without undue burden. The same point is made about claim 7 of claim set A in its amended form (limited to a sequencing reaction). The issue can be decided by reference to claim 12. Claim 7 will stand or fall with it.
245. The argument as explained in the previous paragraph works as a free standing objection. In fact all of MGI’s insufficiency arguments were pleaded as squeezes with obviousness. The way MGI puts the read length argument as a squeeze is clearest in the opening skeleton (para 198). The submission is that the modified nucleotide patents do not disclose anything to suggest to the skilled person that they could achieve read lengths longer than that they would expect could be achieved on the basis of Zavgorodny, even in respect of the exemplified embodiment, let alone across the scope of the claims. The reason MGI put it as a squeeze is I think because it has not sought to call evidence directly to show how difficult it may or may not be, starting from the patent, to carrying out sequencing. Rather it relies on Prof Marx’s evidence about the quality of the data in the patent itself.
246. This first issue is meant to be MGI MNP Issue 5. Although issue 5 is drafted more broadly, the only specific point raised is the read lengths question. MGI MNP Issue 5 also refers to claim 1 but no such point was pleaded nor was it advanced in the closing. I will not allow it to be raised now.
247. The second issue is the submission that claim 12 “covers methods of sequencing using nucleotides, linkers and labels that would not enable the skilled person to perform a sequencing method across the breadth of the claim without undue burden”. This way of putting it is MGI MNP issue 2. It is put in a more specific way in MGI’s opening skeleton at paragraph 199. There it is said that the claim covers nucleotides which could be modified in ways which would prevent their incorporation and covers linkers for which the conditions required for cleavage would damage DNA. I will call this the impractical linkers point, recognising it is not in fact so limited.
248. For both arguments MGI relied on Regeneron v Kymab. Aside from relying on the principles in that case, part of MGI’s point was to suggest that these insufficiencies were examples of the problem which arose in Regeneron of a claim covering later developed successful techniques which techniques could not have been arrived at just with the patent (and the common general knowledge) but needed further steps too to make them work. MGI submitted that the fact that today it is possible to read substantial lengths of sequence using azidomethyl 3' blocker nucleotides with suitable linkers, polymerases and labels etc. does not mean that the patent is enabling.
249. Illumina did not agree with these submissions, arguing that they failed on the facts and involved a mis-application of Regeneron. I will start with Regeneron.
250. MGI focussed on the propositions that Lord Briggs derived from his review of the authorities. The propositions make frequent reference to product claims, but the targets of MGI’s insufficiency attacks to which it contends Regeneron is applicable are process claims. Illumina did not agree that the principles could be transposed in the way MGI contended for. To resolve this I will turn to the context in which these principles were enunciated.
251. Regeneron was concerned with the sufficiency of a product claim. The claim was to a transgenic mouse. The utility of the transgenic mice was their ability to generate a diverse variety of chimeric antibodies. By the time the case reached the Supreme Court, it was not in dispute that the relevant claim extended to a range of types of mice. The different types were characterised by the number of different human antibody gene segments which had been inserted into the mouse, replacing murine gene segments. For example there could be from 1 to 125 of one kind of gene segment (human heavy chain variable segments). The claim claimed a range of types of mice. The range encompassed mice with all 125 of those segments and also any number of segments including just a single segment on its own. The relevance of having a high number of segments was that a larger repertoire of segments gives much greater diversity of antibodies. The patent’s idea of replacing certain mouse antibody genes in situ was called the “Reverse Chimeric Locus”. It solved a problem of immunological sickness in the mice which would otherwise have occurred and which had hampered the ability to generate antibody diversity.
252. The defendant’s transgenic mouse had the whole of the human gene locus inserted in it (so had all 125 heavy chain variable segments) and had been held to infringe. The problem was that as a matter of fact the only thing a skilled person could make based on the patent’s disclosure and the state of knowledge at the relevant time was a mouse with a few segments. This was a very small part of the range, and was the least beneficial part of the range denominated by the number of human variable segments incorporated (paragraph 58). The skilled person at that date could not make a mouse with the whole of the human variable gene locus inserted in it. That came later. The transgenic mice which incorporated the whole locus did benefit from the Reverse Chimeric Locus idea and so in that sense it could be said that what the invention contributed had indeed led to the ability to make a mouse with the whole of the human variable gene locus inserted in it. The Court of Appeal had held the claim was therefore sufficient. However the Supreme Court held (Lady Black dissenting) that the claim was insufficient because it was not possible at the time to make the type of mice claimed which had more than a few human segments inserted.
253. Following his review of the authorities, at paragraph 56 Lord Briggs summarised the principles as follows:
“i) The requirement of sufficiency imposed by article 83 of the EPC exists to ensure that the extent of the monopoly conferred by the patent corresponds with the extent of the contribution which it makes to the art.
ii) In the case of a product claim, the contribution to the art is the ability of the skilled person to make the product itself, rather than (if different) the invention.
iii) Patentees are free to choose how widely to frame the range of products for which they claim protection. But they need to ensure that they make no broader claim than is enabled by their disclosure.
iv) The disclosure required of the patentee is such as will, coupled with the common general knowledge existing as at the priority date, be sufficient to enable the skilled person to make substantially all the types or embodiments of products within the scope of the claim. That is what, in the context of a product claim, enablement means.
v) A claim which seeks to protect products which cannot be made by the skilled person using the disclosure in the patent will, subject to de minimis or wholly irrelevant exceptions, be bound to exceed the contribution to the art made by the patent, measured as it must be at the priority date.
vi) This does not mean that the patentee has to demonstrate in the disclosure that every embodiment within the scope of the claim has been tried, tested and proved to have been enabled to be made. Patentees may rely, if they can, upon a principle of general application if it would appear reasonably likely to enable the whole range of products within the scope of the claim to be made. But they take the risk, if challenged, that the supposed general principle will be proved at trial not in fact to enable a significant, relevant, part of the claimed range to be made, as at the priority date.
vii) Nor will a claim which in substance passes the sufficiency test be defeated by dividing the product claim into a range denominated by some wholly irrelevant factor, such as the length of a mouse’s tail. The requirement to show enablement across the whole scope of the claim applies only across a relevant range. Put broadly, the range will be relevant if it is denominated by reference to a variable which significantly affects the value or utility of the product in achieving the purpose for which it is to be made.
viii) Enablement across the scope of a product claim is not established merely by showing that all products within the relevant range will, if and when they can be made, deliver the same general benefit intended to be generated by the invention, regardless how valuable and ground-breaking that invention may prove to be.”
254. It is clear from Lord Briggs’ judgment as a whole that the principles being considered were not limited to product claims. That is why his review of the authorities drew on claims of all kinds (see e.g. paragraph 37). In my judgment the reasoning in Regeneron is not limited to product claims.
255. However when it comes to specifics, the sub-paragraphs above are clearly focussed on product claims. For example principles (ii) and (viii) are of particular importance in Regeneron because (ii) defines the contribution to the art in such a case as being the ability to make the product itself and (viii) deals with consequences. One can see why this was the case given the issue in Regeneron but in other cases, even about product claims, there may be a different kind of insufficiency alleged for which the ability to make a product will not be the relevant technical contribution. In some cases the products are easy enough to make but where patent claims fall down is because the (alleged) property of those products, which was said to be the thing which made them inventive in the first place, was not shared by all the products within the claimed range. The products could be made alright but they do not do what is promised and so the claim exceeds that technical contribution for that reason. This is Agrevo (albeit Agrevo is a form of obviousness, the same principle applies to sufficiency - Mycogen/Modifying plan cells (T694/92)). It is not what Lord Briggs is talking about in these principles at all, nor do I read these principles as seeking to overturn that line of reasoning. I mention all this simply to illustrate the point that care needs to be taken when transposing these the principles summarised by Lord Briggs in one context in order to apply them to different circumstances.
256. With this in mind, principles (i) and (iii) identified by Lord Briggs are general in nature and apply to any case. To recast principle (iv) as applicable in general terms I have replaced “make” with “perform” from s72(1)(c) of the 1977 Act (bearing in mind that for a product claim in a case like Regeneron perform means make) and made some other consequential changes. Instead of “performed” one could use “carried out” based on Art 83 EPC but nothing turns on the difference. The result is:
iv) The disclosure required of the patentee is such as will, coupled with the common general knowledge existing as at the priority date, be sufficient to enable the skilled person to perform substantially all the types or embodiments [ ] within the scope of the claim. That is what, [ ], enablement means.
257. Turning to principles (v), (vi) and (vii), they go together. They all relate to what exactly it is, within the scope of the claim, which has to be enabled. Lord Briggs distinguished two kinds of range within a claim. One kind is described as a “relevant range” and by contrast the other kind is a range denominated by a “wholly irrelevant factor”. For relevant ranges the law as explained by Lord Briggs is that all types or embodiments across the scope of the claim, as that scope is denominated by that relevant range, must be enabled. That is subject to de minimis or wholly irrelevant exceptions. I think the latter exception refers to the second type of range but that may not matter. In any case the result in Regeneron was that the range from 1 to 125 V segments was a relevant range and since most of that range was not enabled, the claim was insufficient. Whereas the fact that one could say that the claim covered mice with different lengths of tail but the patent had not enabled how to do what it taught with mice with all possible lengths of tail, did not matter because tail length was not a relevant range.
258. One can therefore put Lord Briggs’ principles (v) to (vii) in general terms as follows:
v) A claim which seeks to protect products or processes which cannot be performed by the skilled person using the disclosure in the patent will, subject to de minimis or wholly irrelevant exceptions, be bound to exceed the contribution to the art made by the patent, measured as it must be at the priority date.
vi) This does not mean that the patentee has to demonstrate in the disclosure that every embodiment within the scope of the claim has been tried, tested and proved to have been enabled […]. Patentees may rely, if they can, upon a principle of general application if it would appear reasonably likely to enable the whole range […] within the scope of the claim to be performed. But they take the risk, if challenged, that the supposed general principle will be proved at trial not in fact to enable a significant, relevant, part of the claimed range to be performed, as at the priority date.
vii) Nor will a claim which in substance passes the sufficiency test be defeated by dividing the […] claim into a range denominated by some wholly irrelevant factor, such as the length of a mouse’s tail. The requirement to show enablement across the whole scope of the claim applies only across a relevant range. Put broadly, the range will be relevant if it is denominated by reference to a variable which significantly affects the value or utility of the product or process in achieving the purpose for which it is to be performed.
259. I will return to this after dealing with the other cases but at this stage it is worth saying something about the difference between ranges relevant in the Regeneron sense and other ranges. That is referred to in principle (vii). The identification of the purpose which will be taken into account in distinguishing between the two kinds of range will be a matter of construction of the specification through the eyes of the skilled person imbued with the common general knowledge. The product or process whose utility or value is to be considered will be the claimed product or process. Nevertheless Regeneron shows that one cannot approach this in a simplistic way. The relevant range in that case, the number of segments, is not something expressly called for by the claim in the sense of there being words like “from 1 to 125 V segments”. The range was identified by an exercise of construction of the language used. The term “range” itself as used in Regeneron has a wide meaning. The tail length point simply arises from considering the scope of the term “mouse” in the claim.
260. There is no need to attempt to recast principles (ii) and (viii) since although they are critical to the outcome of Regeneron itself (and would matter in another similar product claim case) they relate most specifically to the facts of Regeneron.
261. I turn to address two other issues of principle relating to insufficiency which need to be considered. They are undue burden and descriptive or functional limitations. The points interact and once I have dealt with them it will be necessary to come back to Regeneron to pull it all together.
262. Illumina pointed to the references to undue burden in the way both of MGI’s issues are advanced (see e.g. Grounds of Invalidity para 3(d)) and submitted that nothing in Regeneron was concerned with that. Illumina also referred to Mentor v Hollister [1991] FSR  577
577 and [1992] RPC 1. In that case the claim required an adhesive to be used to adhere the sheath to the penis. Many adhesives would not have worked but the skilled person could readily select one which would work using reasonable trial and experiment, so the claim was held to be sufficient. Illumina submitted that the patentee is entitled to expect the skilled person to utilise reasonable trial and experiment to implement the claim, including by selecting appropriate examples of individual elements of the claim
and [1992] RPC 1. In that case the claim required an adhesive to be used to adhere the sheath to the penis. Many adhesives would not have worked but the skilled person could readily select one which would work using reasonable trial and experiment, so the claim was held to be sufficient. Illumina submitted that the patentee is entitled to expect the skilled person to utilise reasonable trial and experiment to implement the claim, including by selecting appropriate examples of individual elements of the claim
263. MGI submitted that Mentor was decided before Biogen and to the extent it is inconsistent with Regeneron it has been overruled. MGI also submitted that it is settled law that claims cannot be limited simply to those embodiments which work, citing the contact lens case Novartis v Johnson & Johnson [2010] EWCA 1039.
264. Related to this was a disagreement between the parties about the impact of the German BGH decision Dipeptidyl-Peptidase-Inhibitoren (X ZB 8/12) (“the DPI case”). The case was referred to by Lord Briggs at paragraph 45 as taking the matter no further than Genentech/ Polypeptide Expression (T292/85) or Nabisco/ Micro-organisms (T361/87). Illumina said that unlike Regeneron which was about a claim to products which cannot be made, the DPI case was about a single method of treatment which could be carried out by choosing from a range of “input substances”. MGI said the DPI case was doubted by the EPO in Trustees of Princeton/ OLED (T0544/12) at paragraph 4.9.5.
265. Before going any further I will say that whatever the correct principles are about undue burden, none of them were addressed in Regeneron. No doubt that was because by the time the case reached the Supreme Court, it was on the premise of fact that claimed embodiments (mice) with high numbers of segments could not be made at all at the relevant date. One might put that another way and say that this meant that those mice could not be made without undue burden, but that does not make any difference.
266. To address all this I will start with Novartis v Johnson & Johnson. In that case the claim was essentially to a contact lens made with any silica hydrogel formulation, defined by its function. The functional definition was essentially that the product would be oxygen permeable. The problem was that the patent did not enable the skilled person to predict whether a contact lens made with any particular silica hydrogel would satisfy the requirements of the claim without actually going to the trouble of conducting clinical testing. Not surprisingly that was held to be unduly burdensome and insufficient. In terms of legal principles, Jacob LJ expressly followed the EPO line of authority on undue burden and functional features, including para 2.2.1 of Unilever/Detergents (T435/91), which was also cited by Lord Briggs in Regeneron at paragraph 37. In other words the legal test is that the whole subject matter must be capable of being carried out without the burden of an undue amount of experiment. In terms of legal principles this is just the same as Mentor v Hollister.
267. What Novartis v Johnson & Johnson is not authority for is the idea that just because the patent left it to the skilled person to select a particular starting material for themselves from a class defined in functional terms, either directly or by carrying out anything which could be called a test, that necessarily made the claim insufficient. Quite the contrary. This is made clear in paragraph 89. There Jacob LJ is contemplating what would have happened if success had been easy to predict. The consequence would have been that the claim was not insufficient. On the facts of that case it would have been obvious but that is another matter. I know MGI say that this present case has the same squeeze. It may or may not but the important thing is not to misread the authorities. The law as laid down in Novartis v Johnson & Johnson is not as simple as saying that claims cannot ever be limited to those embodiments which work. Such claims may or may not pose an undue burden on the skilled person. If they do they will be insufficient. If they do not, then not.
268. Or putting it another way, both Mentor and Unilever / Detergents are and remain authority for the proposition that a claim can define the technical features, such as components, in structural or functional terms, designed to cover only those which will lead to a successful result, provided that does not make carrying it out unduly burdensome for the skilled person. At the risk of repetition, this aspect of the analysis was not relevant to Regeneron at all.
269. As for the debate about the DPI case, the claim was a medical use claim to compounds defined in functional terms (DPI inhibitors) for use in treating a particular disease. The BGH held that this claim was not invalid for insufficiency merely because the compounds were defined in functional terms. Functional definitions are permissible, provided they do not create an undue burden for the skilled person, (paragraph 19):
… it may be admissible to recite a group of substances in a generalised form, even if not all substances that belong to this group are suitable for the purpose of the invention, provided the skilled person is easily able to determine the suitability of the individual substances by experiments …
270. I agree with the BGH. This does not mean it will always be permissible to recite a group of components in a generalised way but it will be if no undue burden is involved in determining the suitability of individual candidates.
271. The BGH went on in paragraph 19 to make the obvious point that using functional definitions for components necessarily has the result that the claim could cover the use of a component which had not been invented yet. They then said that this was not a cause for concern as long as such use was making use of the invention. In Regeneron Lord Briggs recognised this aspect of the DPI case and explained that that put the case on the Genentech/Polypeptide Expression (T 292/85) side of the line.
272. I now turn to the Technical Board of Appeal in Trustees of Princeton/ OLED (T 0455/12). The claim in that case was to an organic LED with a layer which included a molecule that is a phosphorescent organometallic iridium compound. The Board noted that this claim defined the iridium component compound in both structural terms (organometallic) and functional terms (phosphorescent). The problem for the patentee was that the structural definition was to an almost infinite class (para 4.3) and the desired functional result is not achieved by all compounds in the class (para 4.4).
273. The basic principles of law were explained in paragraph 4.2 and were uncontroversial. In summary, components can be defined in functional terms as long as the common general knowledge, or the patent itself, provides the skilled person with sufficient guidance on how to select those compounds. Therefore, as the board explained at 4.5, since not all conceivable compounds within the structural definition possess the function, sufficiency could only be found if the skilled person is able to identify the ones which are phosphorescent without undue burden. I agree.
274. The board then went on to hold on the facts that the claim was nothing more than an invitation to perform a research programme to identify suitable compounds and amounted to an undue burden. Having reached that conclusion on the facts the Board referred to an argument by the patentee that certain structures were disclosed in the patent which provided a basis for identifying phosphorescent iridium compounds. However the Board held that that did not help because the claim was far wider than one limited to a principle based on those structures and covered unrelated iridium compounds, thereby exceeding the technical contribution.
275. This was the stage at which the board referred to the DPI case and in particular to the sentence at the end of paragraph 19 which I refer to above and which Lord Briggs noted at paragraph 45. The Board appears to take the view that a functional definition will be necessarily insufficient simply because, as the BGH noted in the DPI case, such language covers things which have not been invented yet. Stated in such a general way I respectfully disagree with the Board and I note that Lord Briggs did not take that view either. This absolutist approach would strike down all functional language and represent a radical change for no discernible benefit to the public. A functional definition cannot help cover things which are not yet invented. That may not necessarily matter at all. What matters is that the skilled person must be able to put the invention into practice without undue burden. They need to be able to come up with components which will work and, if that involves testing things, that testing must not introduce an undue burden.
276. Now I come back to Regeneron. As mentioned already the descriptive term “mouse” in Regeneron was regard as encompassing a range. By the same token any descriptive or functional language will inevitably cover a variety of things and therefore will encompass what one could call a range. Thus it will be necessary to examine whether such a range is a relevant one in the Regeneron sense. If it is a relevant range then the consequences in Regeneron will follow if it is not enabled across the whole range (subject to de minimis exceptions) and the presence of a type or embodiment within that range which cannot be performed at the relevant date will be fatal even though, if it was able to be performed years later, it could be said to draw on the technical contribution made by the inventors. However if the range is not a relevant range then no difficulty of that kind arises. That is the point Lord Briggs is making at paragraph 42. Separately and in either case, the standard being applied is one of no undue burden.
277. To take an example mentioned in argument in this case, say an inventor invented a new teapot which was inventive and useful because its spout was shaped in a new way so as not to drip. The claim would be to a teapot with the spout shaped in that special way. The claim might well not say anything about the material from which to make the teapot, because it is irrelevant to the invention. Equally the claim might refer to “a tea pot made of any suitable material”. There would be no difference between a claim which expressly said that or one which was silent. Either way the claim can be said to encompass a range of teapots made of different materials. Now the patent needs to enable the skilled person to make the product. In the example I will assume the skilled person could choose, identify and test suitable materials at the priority date without an undue burden. China would work and chocolate would not. However the claim would be infringed later on even if a teapot was made using a new inventive form of Pyrex glass which had not been invented at the teapot patent’s priority date. Furthermore in my judgment this fact, that the claim covers types of teapot which it does not enable, does not reveal some insufficiency. The fact that the skilled person could not make such a teapot at the priority date of the teapot patent does not matter. What does matter is that the descriptive feature of the claim, which is at least implicit in the claim, that the teapot has to be made of a suitable material, is not a relevant range in the Regeneron sense. However note the potential for error here. The material from which a teapot is made is plainly crucial to its function as a teapot. There are materials which are not suitable to use for teapots. That is not the kind of relevance which Regeneron is referring to. Relevance in the Regeneron sense is a much more particular concept which depends on examining all the circumstances, and depends not simply on the invention (that is to say the claim as drafted) but also on what I can only think of calling the essence or core of that invention (closely related to the technical contribution and/or the inventive concept). Although the invention in this example is (by definition) a teapot since that is what is claimed (s125, 1977 Act), nevertheless the value, utility and purpose referred to by Lord Briggs in principle (vii) are concepts which in this example would be focussed on the shape of spout. In fact I doubt this teapot example has much of a relevant range (of spout shapes) at all. On the facts of Regeneron itself the range of numbers of segments was clearly relevant to the essence of the invention since it was the means for getting high antibody diversity, whereas different kinds of mice was not. In other words when applying this test one may need to examine the essence of the invention as well as the claim language itself.
278. Once the concept of a relevant range is properly understood, I think it will be an unusual case in which the kind of ordinary descriptive or functional language one sees in most patent claims will be regarded a relevant range in the Regeneron sense.
279. In summary, the principles I derive from these authorities are:
i) When examining any aspect of claim scope for the purposes of the enablement it is necessary to distinguish between ranges relevant in the Regeneron sense and other ranges.
ii) For ranges relevant in the Regeneron sense, to be sufficient, there must be enablement across the whole scope of the claim within that relevant range (subject to de minimis exceptions) at the relevant date. If a type or embodiment within such a range is not enabled at that date then the fact it could be made later, as a result of further developments not enabled by the patent, even though it never could have been made without the invention, will not save the claim from insufficiency.
iii) Not all claims will necessarily contain a range relevant in the Regeneron sense but if they do, then this principle applies to that range.
iv) An example of an other range, not relevant in the Regeneron sense, will be a descriptive feature in a claim (whether structural or functional) which can cover a variety of things, but for which that variety does not significantly affect the value or utility of the claimed product or process in achieving its relevant purpose. The relevant purpose is judged in all the circumstances, starting from the terms of the claim itself but also, where appropriate, by reference to the essence or core of the invention.
v) For a claim feature which amounts to a range in this other sense, the skilled person must still be able to make a suitable selection, without undue burden, in order for the claim to be sufficiently disclosed. However provided that is so at the relevant date, such a claim feature will not be insufficient simply because it is capable of also covering within its scope things which had not been invented at that relevant date.
vi) When examining enablement of any kind, the test is always about what the skilled person is able to do without undue burden. The patentee is entitled to expect that the skilled person, in seeking to make the invention work, will exercise that skill. If need be that exercise will involve testing and experiments, as long as it is not unduly burdensome.
280. Turning to the facts, I will address impractical linkers first.
Impractical Linkers
281. The relevant features of claims 7 and 12, as dependent on claim 2, are the terms nucleotide, polymerase, linker and detectable label. Their significance is that for each of them it is possible to conceive of impractical versions. Strictly one would need to do the experiment to check but it is not hard to imagine a nucleotide molecule with certain extra substitutions, say at the 4' or 2' position, which would not work. The claim does not expressly prohibit any such substitution. Unsurprisingly Prof Leadlay agreed with Prof Marx that it was possible to make additional modifications to the nucleotides beyond the 3'–O–azidomethyl group that would prevent their being incorporated by the polymerase and so make them unsuitable for sequencing. However Prof Leadlay could not see why the skilled person would make such modifications and his view was that the skilled person seeking to make the method work would avoid such modifications.
282. The facts are essentially the same for polymerases. Prof Marx and Prof Leadlay agreed that not all polymerases would be capable of incorporating the 3'–O–azidomethyl nucleotides but Prof Leadlay’s view was that known polymerases were capable of doing so and a skilled person seeking to make the method work would be able to select such polymerases.
283. On linkers there was a slight difference. Prof Marx gave evidence that some of the linkers listed in the patent specification as cleavable linkers would require conditions for cleavage which were known to be capable of damaging DNA (e.g. acidic or oxidative conditions). The reference to cleavage conditions is at paragraph [0076] in the 289 specification. Prof Leadlay did not accept that the cleavage conditions described in the patent were necessarily so severe as to be incompatible with the sequencing method. However again his view was that in practice the skilled person, if they were concerned about specific conditions, would simply choose a linker which could be cleaved in milder conditions and would be able to do so without difficulty.
284. In this context the evidence did not, as far as I am aware, focus on the detectable labels but I doubt it makes any difference.
285. My findings on the facts are the same for each of these claim features. For nucleotides, there are additional modifications to the nucleotides beyond the 3'–O–azidomethyl group that would make them unsuitable for use in the claim methods of claims 7 and 12. However, based on Prof Leadlay’s evidence, I find that the skilled person seeking to make the method work would avoid such modifications without any undue difficulty. For polymerases, again there will be polymerases that do not work but the skilled person, given the information in the patent and the common general knowledge, will be able to select suitable polymerases without difficulty.
286. For linker cleavage conditions there is a minor point of interpretation of the specification. The specification asserts that the linker can be cleaved “by any suitable method”. The mention of acidic and oxidative conditions is in a list which includes basic and reductive conditions too and other features. There will be reaction conditions within the generality of the term “acidic” which would be unsuitable, although I do not accept that the patent positively asserts that any acidic or oxidative conditions of any sort will be suitable. What matters in the end is that the skilled person would, I find, be able to choose suitable combination of linker and cleavage conditions without difficulty.
287. If a finding of fact is required for detectable labels, I would reach the same conclusion. No undue difficulty is presented to the skilled person to select a suitable label.
288. What are the legal consequences of this? The first question is whether the variety of suitable things covered by these terms amount to relevant ranges in the Regeneron sense. If they do then the claim would be insufficient irrespective of the absence of an undue burden putting them into practice. However in my judgment none of these terms amounts to a relevant range in the Regeneron sense. The different instances within these “ranges” are not of the essence of the invention. The essence of the invention in claim 12 is a sequencing method whose utility derives from the use of a 3'-O-blocked nucleotide. Plainly the particular nucleotide, polymerase, linker, label and cleavage conditions chosen have to be suitable. However beyond the simple fact of being suitable, their individual type does not significantly affect the value of the method to achieve the purpose for which it is being carried out. The same applies to claim 7. Therefore the fact that, for example, the claim would cover a method performed using a later invented component, does not matter.
289. I wondered if (as Illumina submitted) there was a simpler answer to this Regeneron point in that the ranges encompassed are all to be construed implicitly as limited only to those components which are suitable (“suitable nucleotides” etc.), however I do not believe that would be an answer, since it does not face up to the fact that the claim also covers later invented suitable types.
290. However just because the claims are not invalid based on the Regeneron principle is not the end of the analysis. Part of MGI’s case, based as it is on a squeeze on inventive step, is that the need to select suitable types presents an undue burden for the skilled person. I reject that. On the facts I have found the skilled person can select suitable types of the various components without difficulty. The exercise of skill and some routine testing may be needed but I am not satisfied any burden of undertaking that work is undue. I reject this limb of the insufficiency.
291. Moreover there is no squeeze with inventive step. The position of the skilled person is entirely different from the position they were in based on the prior art. Taking polymerases as an example, the fact that Metzker 1994 showed inconsistent results with a variety of the polymerases and blocking groups tested in that paper is not evidence that, armed with the patent, the skilled person has an undue difficulty selecting suitable polymerases to make the invention work with 3'–O–azidomethyl blocked nucleotides. I reach the same conclusions for linkers, cleavage conditions and detectable labels.
Read length
292. The first question is whether in claim 12 a read length from a single base up to whatever upper limit one chooses, is a relevant range in the Regeneron sense. MGI submitted that it was, Illumina in effect submitted that it was not. One of Illumina’s submissions was that the claim was to a method (singular) for determining a sequence rather than to a range of methods.
293. I start with the claim language. Claim 12 relates to a method of sequencing at least one nucleotide. It does not matter how many are sequenced, as long as one nucleotide is sequenced. The claim would be infringed if a person sequenced 100 nucleotides, but not because 100 is part of a claimed range. The reason sequencing 100 nucleotides infringes is because the sequencer would inevitably sequence one nucleotide when they did so. The sequencing of the 100th nucleotide after 99 previous ones is not what makes the activity an infringement. In my judgment, as a matter of construction this claim is not a claim to a range of read lengths.
294. In any case, in terms of purpose, the fact that, no doubt, the skilled person would usually like to sequence as many nucleotides as they can is not the issue. Read length is not a variable which significantly affects the value or utility of the claimed process in achieving the purpose for which that process is to be performed. The purpose of sequencing the one nucleotide is to determine the identity of that nucleotide. The determination of the identities of other nucleotides apart from that one is irrelevant. It cannot be said that sequencing more than one nucleotide is the essence or core of the invention of claim 12 either.
295. Since the patent clearly enables sequencing at least one nucleotide (of any relevant sort - G, C, A or T) then there is no Regeneron insufficiency here. The same conclusion applies to claim 7.
296. At this point I will track back to obviousness because MGI contends this is a squeeze and so if I reach the answer I have reached, then that could only be because the claim should have been obvious. I do not agree with that, for the following reasons.
297. MGI is right that this conclusion highlights that all that needed to be obvious to invalidate claim 12 was to sequence a single one nucleotide using the 3' azidomethyl blocked nucleotide (with linker etc.). (And note that there is a bit more to claim 12 and claim 7 than incorporation - about blocking a subsequent nucleotide in claim 7 and removal of a label before incorporation of another nucleotide in claim 12, but nothing turns on those points.) The problem for MGI is that this dimension to the arguments on inventive step does not help MGI on the facts. The conclusion rejecting insufficiency is not inconsistent with the finding of non-obviousness.
298. What MGI is really trying to say is that because (say) Metzker 1994 did show the sequencing of one nucleotide, it follows that there is no real technical advance in this case because the claim only requires the sequencing of one nucleotide. Or similarly, that it is not open to hold (as I have) that the common general knowledge of the skilled person was that the technique of sequencing by synthesis using reversible chain terminators may well be something which could not be made to work, among other reasons because it was not reliably repeatable, because all that one needs to do to make the claimed invention work is sequence one nucleotide, and that was feasible based on Metzker. Neither point is right. There is a technical advance for the reasons already referred to. Essentially, the azidomethyl blocking group does meet the stringent requirements referred to in the patent. As for the common general knowledge, the state of the common general knowledge is a matter of fact unaffected by the scope of the claim. The fact that armed with the patent the skilled person only has to sequence one nucleotide to satisfy claim 12 does not mean the common general knowledge changes. Nor does it, in fact, alter the reasons why an azidomethyl blocking group was not obvious over Zavgorodny.
299. Given that Regeneron is a new development of the law of insufficiency I will also briefly consider what the situation would be if a read length of more than one was a relevant range in the Regeneron sense. I am quite satisfied on the evidence I have heard that the skilled person armed with the patent, willing to make the invention work, would be able carry out repeatable sequencing by synthesis using a 3'–O–azidomethyl blocking group. They would be able to run the sequencing process for substantially more cycles that the few shown in the gels in the patent. It would require the optimisation work described by Prof Leadlay and I have accepted his evidence. There would be tests to perform and a lot of work but none of it would represent an undue burden. The professor was not asked where a limit might be and MGI did not set out to establish that. MGI’s sole case on the evidence was based on Prof Marx’s criticisms of the quality of the data in the patent but as I have explained already, I do not accept them. Nor do I accept that those concerns indicate that the skilled person would be hampered in being able to put the sequencing technique disclosed in the patent into practice.
300. The legal burden of proof to establish insufficiency is on MGI. No evidential burden has shifted. There is no evidence which would allow me to make a finding on the balance of probabilities that the limit of what the skilled person is enabled to do, without an undue burden, is anything less than what they would reasonably regard as what had been promised by the patent, or claimed if, contrary to my finding, claim 12 does represent a range of read lengths in the Regeneron sense.
301. I reject MGI’s case based on insufficiency.
Added matter - amendment
302. No amendment will be allowed if it has the result that the matter disclosed extends beyond that disclosed in the application for the patent as filed. The leading case on added matter is Nokia v IPCom [2012] EWCA Civ 567. At paras 45-60, Kitchen LJ reviewed the law on added matter. At para 60 he described the key question as “whether the amendment presents the skilled person with new information about the invention which is not directly and unambiguously apparent from the original disclosure. If it does then the amendment is not permissible”.
303. The issue relates to claim 9 (claim set B) of 289 (claim 13 as granted). Claim 9 adds to the sequencing method claims of 289 (claim 6 of claim set B et al) a requirement to remove the blocking group using a water soluble phosphine. Illumina seek to amend that claim to add the words “under neutral, aqueous conditions”. MGI says this amendment adds matter. The reason why is a bit of a paper chase. It is true (as Illumina points out) that there is textual support for the amendment at p43 ln 10 of the application as filed (this happens to be the same text about removing the azidomethyl group which has been quoted above from the priority document P2). The same passage also seems to have become para [0103] as granted in 289. However MGI says that in the application as filed there was also a limiting definition of an aqueous solution. It was at p5 ln 26-34. The definition places a lower limit on the amount of water in the liquid of at least 20%. This definition has not been carried forward into the granted patent. Therefore, it is said by MGI, whereas in the application as filed “aqueous conditions” would be understood to be at least 20% water, as amended the claim will add matter because it uses the general phrase and could be understood to envisage aqueous conditions with less than 20% water.
304. Neither party devoted much effort to this point but it still needs to be decided. No evidence has been drawn to my attention which is said to have a bearing on the issue. I say this because I believe some evidence was required. While the definition passage which MGI relies on is indeed not in the specification of 289 as granted, that passage is in the specification of  578
578 (at paragraph [0015]), which of course is also based on the same application as filed. Its context (as it is in the application as filed) it is part of a section (from paragraph [0014] as granted of
(at paragraph [0015]), which of course is also based on the same application as filed. Its context (as it is in the application as filed) it is part of a section (from paragraph [0014] as granted of  578)
578) which is addressing what is said to be a method of deprotection using a water soluble transition metal catalyst. Perhaps that is not relevant to the claimed method (I do not know but there are notably fewer references to transition metal complexes in 289) but it would then explain why the passage was deleted (presumably without objection from the examiner) when the 289 specification was drawn up. Furthermore if MGI’s objection to the claim amendment was a good one, then that removal from 289 of that passage discussing transition metal catalysts has had the consequence that the other passage which refers to aqueous conditions to deprotect the azidomethyl (now at [0103] of 289) adds matter for the very reason MGI objects to the amendment. In other words if the argument is right the specification itself adds matter irrespective of the claim amendment. Of course that does not mean the argument is necessarily wrong but it puts it in context.
which is addressing what is said to be a method of deprotection using a water soluble transition metal catalyst. Perhaps that is not relevant to the claimed method (I do not know but there are notably fewer references to transition metal complexes in 289) but it would then explain why the passage was deleted (presumably without objection from the examiner) when the 289 specification was drawn up. Furthermore if MGI’s objection to the claim amendment was a good one, then that removal from 289 of that passage discussing transition metal catalysts has had the consequence that the other passage which refers to aqueous conditions to deprotect the azidomethyl (now at [0103] of 289) adds matter for the very reason MGI objects to the amendment. In other words if the argument is right the specification itself adds matter irrespective of the claim amendment. Of course that does not mean the argument is necessarily wrong but it puts it in context.
305. I am not satisfied this amendment amounts to added matter for three reasons. First, for the argument to succeed it would have to be established that the skilled person (with the common general knowledge) would actually think that “neutral, aqueous conditions” disclosed the idea of conditions in which the water content was less than 20%. That would require evidence. There is none. Second it must be remembered that coverage is not the same as disclosure of information. Even if the claim could be said to cover solutions with less than 20% water, I am not satisfied there is a disclosure of such a solution by the amendment. Third I do not accept that the skilled reader of the application as filed would necessarily think that the definition given in one context was necessarily relevant to the reference to neutral, aqueous conditions in another context.
306. Thus the amendment is formally allowable. However as far as I am aware the purpose of the amendment has not been drawn to the court’s attention. The pleaded reasons are entirely generic and opaque. I guess part of the reason for the amendment is so that the claim is in the same form as a set of claims before the EPO in the continuing opposition proceedings. That all very well (and is the right thing to do) but the court ought not be in doubt about what the consequence would have been if the amendment was not formally allowable. Presumably there is somewhere an attack on validity to which this is an answer. No amendment should be permitted without the patentee explaining with reasonable specificity what the purpose of the amendment is. I will allow Illumina the opportunity at the hearing to determine the form of order to explain what the purpose is, and if that is done satisfactorily then I will allow the amendment.
Infringement
307. As explained in the introductory section above, infringement of a number of claims is admitted. This section is only concerned with the infringement issues where a point has to be decided. To recap, they are (i) whether Cool MPS falls within claim 12 of  578
578 (Claim set A) [MGI MNP Issue 10] and (ii) whether either the two colour variant of Standard MPS or the DNBSEQ E method fall within the kit claims, claim 20 of
(Claim set A) [MGI MNP Issue 10] and (ii) whether either the two colour variant of Standard MPS or the DNBSEQ E method fall within the kit claims, claim 20 of  578
578 (claim set A) or claim 4 of 289 (claim set B) [MGI MNP Issue 9].
(claim set A) or claim 4 of 289 (claim set B) [MGI MNP Issue 9].
308. Illumina advances its case of infringement both on a normal construction and (for issue (i) about Cool MPS) by the doctrine of equivalents. Following the Supreme Court’s decision in Actavis v Eli Lilly [2017] UKSC 48, the correct approach to infringement by equivalents is to ask three questions (see [66] of Lord Neuberger’s judgment in Actavis):
(i) Notwithstanding that it is not within the literal meaning of the relevant claim(s) of the patent, does the variant achieve substantially the same result in substantially the same way as the invention, ie. the inventive concept revealed by the patent?
(ii) Would it be obvious to the person skilled in the art, reading the patent at the priority date, but knowing that the variant achieves substantially the same result as the invention, that it does so in substantially the same way as the invention?
(iii) Would such a reader of the patent have concluded that the patentee none the less intended that strict compliance with the literal meaning of the relevant claim(s) of the patent was an essential requirement of the invention?
309. To establish infringement the answers have to be yes, yes and no.
310. On equivalents, the only issue is the application of the third question. It is common ground that in both cases the first two questions are to be answered in the affirmative.
(i) whether Cool MPS falls with claim 12 of  578
578 (Claim set A) [MGI MNP Issue 10]
(Claim set A) [MGI MNP Issue 10]
311. Claim 12 (claim set A) is to a method for determining a sequence. One of the steps called for is the incorporation of a nucleotide defined in claims 2 to 5. It is convenient to consider the issue by reference to claim 2 dependent on claim 3. In other words what is required is incorporation of a nucleotide to which a detectable label is linked by a cleavable linker.
312. In Cool MPS, the process involves using 3'-O-azidomethyl blocked nucleotides to synthesise the strand complementary to the target sequence. In the form when the polymerase acts on them, the nucleotides do not have a label attached (nor a cleavable linker). The next step is that detector antibodies are introduced. These antibodies have detectable labels. There are four kinds of antibody/label conjugate in order to detect the four kinds nucleotide. Thus the antibody which binds to a 3'-O-azidomethyl blocked nucleotide in which the base is A has a different label from the antibody which binds to the one with base C, etc. Once the antibody binding step has been carried out, the excess antibodies are washed off and the detection step occurs. When the detection takes place the labelled antibody is bound to the 3'-O-azidomethyl blocked nucleotide in the chain.
313. Thus MGI says that the nucleotide in the state it is incorporated into the chain does not have a linker or label attached to it. Therefore there is no incorporation of the required thing in the claim. By contrast Illumina says that if one considers the state of the system when detection takes place, the antibody has bound to the nucleotide and that whole conjugate structure has been incorporated. In that conjugate structure the nucleotide is linked by the antibody to a label. Therefore since the required thing in the claim has been incorporated, the claim is satisfied. Illumina also says that if this is not a literal infringement it is equivalent and infringes that way.
314. MGI’s second point is that even if that composite structure is to be regarded as incorporated, there is no infringement because the claims require the cleavable linker to be covalent in nature and to be linked only to the base. As regards the first aspect, there is no dispute that antibody binding is not covalent and so, if MGI is right on construction, then there is no literal infringement. However Illumina disagrees with that construction, submitting the claim is not limited to covalent linkers, and also again relies on equivalence even if there is no literal infringement. The other point arises because the antibody binds to more parts of the molecular structure than the base. The issue is a pure point of claim construction. There is no equivalents aspect.
315. I will start with both aspects of the linker issue.
Cleavable linker
316. The terms “linker” and “cleavable linker” are not terms of art. Therefore their meaning is a question of construction of the patent specification. Prof Marx’s firm view, that a linker had to covalently connect two entities, is not relevant.
317. MGI’s case is that all the linkers in the specification are covalent in nature and so, the skilled reader would see that what the patent meant by the term was a covalently bonded chemical group. I agree with MGI that all the examples given in the specification are covalent (e.g. the last two sentences of [0023], and the linkers in [0079]-[0090] (using the numbering in 289)). However I do not agree that that leads to the conclusion that the skilled reader would think that a characteristic about the nature of the chemical bonding had to be read into the term. The skilled reader would understand the expression “linker” as a general one and one which is defined functionally. It is anything which links. No doubt, as the skilled reader would see from the patent, the examples the patentee could think of when the patent was written were indeed covalent but the reader can also see that the patentee has used the broadest language available to define that which links the label to the base. There is no basis for assuming the patentee used language in a manner intended to exclude anything which in fact makes a suitable link between the base and the label.
318. There was a point about the passage in the specification about multi-component linkers and biotin (paragraph [0066] of 289). Illumina suggested it referred to non-covalent structures like biotin. However there is a bit more to it than that. Here the patent is indeed contemplating what it calls multi-component labels, and in those instances the detection of the label moiety occurs by non-covalent binding of a detector molecule to a label molecule. One example given is using a streptavidin molecule to bind to a biotin label. However there is nothing here to suggest that the mode of attachment of the biotin label moiety to the base is any different from any other teaching in the patent. This paragraph of the patent does not assist Illumina (nor does the reference in this paragraph to antibody detection at the end of it, which would be understood in the same way).
319. MGI relied heavily on the passage at paragraph [0076] (of 289) which states that cleavable linkers are known in the art and says that conventional chemistry can be applied to attach a linker to a base. This does not help. Prof Leadlay’s view (to the extent it is relevant) was that this would be understood very often to be covalent attachment but did not absolutely exclude other ways of doing it. I accept that.
320. Therefore, turning to Cool MPS, I find that when the detector antibody binds to the 3'-O-azidomethyl blocked nucleotide which is itself bound to the complementary target nucleotide in the sequencing reaction, a molecule of claim 3 is formed. The fluorescent label is linked to the base part of the nucleotide by a cleavable linker (consisting of the covalent linker linking the label to the antibody and the antibody itself linking that to the base).
321. I turn to the further point arising from the fact that the antibody will not simply bind to the base but will bind to other parts of the molecule too, including the 3' azidomethyl blocking group. That is not excluded by claim 3. The claim simply requires the base to be linked to the label by the linker. As long as that is satisfied, other interactions or links are not positively excluded. The fact that as granted what is now claim 3 (claim set A, which was claim 7 as granted) had a counterpart claim which referred to linking the detectable label through the blocking group (claim 8 as granted) makes no difference.
322. The question of equivalents does not therefore arise however I will consider it on the hypothesis that, on the contrary, the term cleavable linker does require a covalent bond. MGI suggested that the skilled reader would take it that the patentee was aware of and contemplated antibody detection but did not claim it. I do not agree that that is a fair way of putting it. MGI here is relying on the same paragraph [0066] (of 289) which refers to biotin and antibody detection. As MGI was at pains to point out when considering normal construction, this paragraph is not concerned with alternatives to the conventional kinds of linkers described elsewhere. The non-covalent interactions here, between the streptavidin detector and biotin label or between the antibody detector and dinitrophenol label, are not examples of non-covalent linkers. Therefore they would be within the claim even if it was limited to covalent linkers.
323. Conversely the reference to antibody detection in that paragraph does not assist Illumina either for the same reason. It is different.
324. MGI also suggested that the skilled person would think strict compliance with the literal meaning of the expression (construed to mean covalent) was essential because the skilled person would know that if they wanted to use an antibody detection technique like Cool MPS they would need to raise the antibodies themselves. I do not buy that. There is nothing in the specification which would lead the skilled to think that the reason for limiting the linkers to covalent linkers was anything to do with that sort of consideration. As for the common general knowledge, as between Prof Leadlay’s evidence that the team could be confident of raising a suitable antibody and Prof Greenberg’s view that antibodies for DNA lesions did not work well, I prefer Prof Leadlay on this. I do not accept MGI’s suggestion that Prof Leadlay’s evidence was bombastic. Prof Greenberg was not an expert on the relevant antibody literature.
325. I cannot see any good reason why the skilled person would think strict compliance was essential and so, if the term is limited to covalent linker, I would find infringement by equivalence.
Incorporation of a nucleotide [of claim 3]
326. To recap, in Cool MPS at the moment in time when the unlabelled 3'-O-azidomethyl blocked nucleotide joins the chain complementary to the target sequence it is not a molecule of claim 3. Therefore MGI says there is no incorporation of the required molecule. By contrast at the moment in time when the fluorescent detection takes place, the relevant nucleotide is a molecule within claim 3, because the base is linked to a detectable label via a cleavable linker. Therefore Illumina says the claimed molecule has been incorporated, albeit in two steps. Which is the right way of looking at it?
327. The term “incorporation” is given a wide meaning in the patent. Paragraph [0055] of 289 provides simply that it means “becoming part of” the relevant nucleic acid (eg DNA) molecule.
328. MGI says the claim refers to incorporation of labelled nucleotides (e.g. paragraph [0004]). So it does but I do not agree that that resolves the distinction between the two parties’ cases.
329. MGI also points to passages (e.g. [0028]) which describe the thing being acted on by the polymerase as the blocked and labelled nucleotide (my emphasis). MGI also submits that the patent explains that the linker should be long enough so as to hold the label far enough away so as not to interfere with the polymerase enzyme (paragraph [0088] of 289). These points are right and they reflect the specifics of the methods exemplified in the patent. The question is whether they justify reading a limitation into the claim. They would be stronger if the claim as expressly stated that the process of incorporation had to be completed by the polymerase alone, but the claims say no such thing. The polymerase itself is not even mentioned in either claim 7 or claim 12 (claim set A).
330. MGI also points out that the patent indicates that it is desirable that the cleavable linker should not interfere with subsequent incorporation of a labelled nucleotide (paragraph [0068] of 289). However, although I do not think it matters, in case it does I do not accept that MGI has established that there is no risk of interference with the subsequent nucleotide in the sequencing effort using Cool MPS. That is because the Cool MPS process contains a step which only makes sense if there is an appreciable risk that some antibodies remain bound even after the washing step to remove them. That step is a step to cleave the covalent linkage between the fluorescent label and the antibody.
331. The experts (Prof Marx and Prof Leadlay) stated how they understood the claims but as with the point on linker, the term incorporation is not a term of art. This point of construction is a matter for the court, adopting the mantle of the skilled person.
332. MGI points out that claim 12 (claim set A) is a method claim not a product claim, which is right of course. MGI submits that what matters, as a matter of process, is what the polymerase enzyme incorporates into the growing complementary polynucleotide. I do not accept it is that simple. When one focusses on the process steps required by the claim, there are three steps in the overall method, and they are all important. They are (i) the incorporation of a nucleotide of claim 3, (ii) the determination of the identity of the incorporated nucleotide of claim 3, and (iii) the removal of the blocking group and the label before the incorporation of the next complementary nucleotide. The overall method is a way of determining the sequence of a target single stranded polynucleotide by monitoring the sequential incorporation of complementary nucleotides.
333. In Cool MPS the incorporation of an unlabelled 3'-O-azidomethyl blocked nucleotide by the polymerase does not, on its own, satisfy step (i) but once the labelled antibody has bound to that nucleotide and then detection takes place at step (ii), it is entirely apt to say that a molecule of claim 3 has become part of the relevant strand. It has been incorporated. The reason why that is so is because the incorporation of the molecule of claim 3 took place in two steps. The first step was to make a new phosphodiester linkage and the second was to link the blocked molecule to an appropriate label. Read as a whole, there is nothing to limit claim 12 so as to exclude a method in which the incorporation takes place in two steps. Therefore Cool MPS infringes.
334. As before I will consider equivalents in case I am wrong. MGI submitted that one should not approach the third Actavis question on the footing that the skilled person must assume that the patentee knew about the proposed variant. I think that must be right in the sense that one does not make an assumption of any kind (unlike the second question), but I doubt anything turns on it.
335. On the hypothesis that the normal construction of the claim requires a single incorporation step of the molecule in the form of claim 3 by the action of the polymerase, MGI’s major submission is that there is no disclosure or contemplation in the patent of an alternative to that approach, and referred to three technical issues associated with that. One is the paragraph [0066] multicomponent label point. That is addressed above and adds nothing. Second is the suggestion, put to Prof Leadlay, that the patent, by referring to attachment of the label to the base or the blocking group, is inviting the reader to think other positions are less favourable. Prof Leadlay accepted that was a reasonable possibility and I accept that evidence. However I do not agree that it means that the patent can be read as actually ruling out those attachments or other kinds of attachment.
336. The third point is about so called “scarring”. This is the fact that after a covalent linker is cleaved, part of the linker moiety remains attached to the nucleotide and might interfere with subsequent steps. Prof Marx gave some evidence about this but it was based on material which would not be common general knowledge and I do not accept it represented the thinking of the skilled person.
337. MGI’s argument was that the skilled person would presume that if the patentee had thought of a way of avoiding scarring by not having any sort of attachment to the incorporated nucleotide (and thereby no scarring), then the patentee would have mentioned it and so the fact that it is not mentioned proves the patentee did not think of it.
338. I think the position is simpler than these submissions seek to make it. I agree with MGI that the patentee does not describe an antibody detection method like the Cool MPS method, nor does it describe the idea of a two step incorporation. However I do not agree that this answers the third Actavis question in MGI’s favour. Indeed if the patent had disclosed a two step method but had a claim which on a literal construction excluded it, then that would be a much stronger case for answering the third question in MGI’s favour.
339. Another suggestion was that one might believe that strict compliance was essential to avoid a problem with insufficiency. Put in this very broad way, I do not accept the point. It would apply in every case. If there was something specific (such as the argument about raising antibodies) then that could be a better argument but that one did not succeed on the facts.
340. There is nothing in the specification or the common general knowledge which would lead the skilled reader to think that if (contrary to my finding) the claim literally excludes two step incorporation, then strict compliance with that was essential.
341. Therefore I would find infringement by equivalence if, contrary to my earlier finding, there was no infringement on a normal construction.
(ii) whether either the two colour variant of Standard MPS or the DNBSEQ E method fall within the kit claims, claim 20 of  578
578 (claim set A) or claim 4 of 289 (claim set B) [MGI MNP Issue 9].
(claim set A) or claim 4 of 289 (claim set B) [MGI MNP Issue 9].
342. The point which these infringement issues relate to is that each of the two systems to which this issue relates use the same coding scheme whereby two labels can be used to encoding four types of nucleotide by having one label on one, one on another, two on the third and no label on the fourth. Therefore only three of the types of nucleotide actually carry a label.
343. Claim 20 of  578
578 (claim set A) provides for “A kit, comprising, a plurality of different nucleotides wherein said plurality of different nucleotides are as defined in [claim 3]”. Claim 3 relates to a 3'–O–azidomethyl blocked nucleotide with a detectable label linked to the base by a cleavable linker. MGI contends that claim 20 is not infringed because the claim should be understood as requiring all the nucleotides present to satisfy claim 3 (i.e. have a detectable label) whereas in the two MGI systems only three of the four types do that. I reject that as a ground for non-infringement. As Illumina submits, claim 20 used the word “comprising” which would be understood to mean includes but is not limited to. In other words provided there is a plurality of types of nucleotide which satisfy claim 3 (and there will be three types in either system), it follows that the claim is satisfied. The fact that there is also a fourth type of blocked nucleotide which is unlabelled does not matter.
(claim set A) provides for “A kit, comprising, a plurality of different nucleotides wherein said plurality of different nucleotides are as defined in [claim 3]”. Claim 3 relates to a 3'–O–azidomethyl blocked nucleotide with a detectable label linked to the base by a cleavable linker. MGI contends that claim 20 is not infringed because the claim should be understood as requiring all the nucleotides present to satisfy claim 3 (i.e. have a detectable label) whereas in the two MGI systems only three of the four types do that. I reject that as a ground for non-infringement. As Illumina submits, claim 20 used the word “comprising” which would be understood to mean includes but is not limited to. In other words provided there is a plurality of types of nucleotide which satisfy claim 3 (and there will be three types in either system), it follows that the claim is satisfied. The fact that there is also a fourth type of blocked nucleotide which is unlabelled does not matter.
344. As the encoding scheme shows, it is not necessary to label all types of nucleotide in order to be able to detect all types (as Prof Leadlay said in evidence).
345. The word “plurality” will be interpreted as two or more. The claim would not be satisfied by a kit in which all the labelled nucleotides were the same type, on the argument that the plurality could be satisfied by there being more than one individual nucleotide molecule with a label. The claim is talking about types of nucleotides.
346. By contrast claim 4 of the 289 patent (claim set B) is not infringed. This claim provides for a “kit comprising four modified nucleotide triphosphate molecules, … where each nucleotide has a base that is linked to a detectable label …”. Again in my judgment the claim would be understood to be referring to four types of nucleotide. That is true reading the claim in context and is confirmed by the fact the number chosen is four. The four obviously refers to the four type of bases of DNA (C, G, A and T). Therefore to satisfy claim 4 the kit must include labelled nucleotide molecules for all four types. That is not the case for either system. Each of the two colour variant of Standard MPS and the DNBSEQ E method have only three types of nucleotides with labels attached.
EP 1 828 412 - ascorbate
347. The skilled person relating to the 412 patent is essentially the same as before, a molecular biologist, an organic chemist and a fluorescence chemist. The priority date is 13th December 2004. By now the idea of sequencing by synthesis was part of the common general knowledge of such a team, as a whole. The fluorescence chemist would receive information about it from the other members.
348. The invention concerns using ascorbic acid or a salt thereof in the buffer used at the detection step in sequencing by synthesis, in order to mitigate photodamage to DNA. MGI says it is obvious over the prior art Buechler. Before going further it is convenient to deal now with two issues of principle arising from the relationship between Buechler and the common general knowledge. One of the submissions is that the content of Buechler (see below) adds little or nothing to the common general knowledge. Arising from that Illumina submitted it was relevant to consider the obviousness case over Buechler as amounting to an attack based on common general knowledge alone. This was said to have two consequences. First Illumina referred to the caution in Apimed v Brightwake [2012] EWCA Civ 5 at para 54, citing Abbott v Evysio [2008]  EWHC
EWHC 800 (Pat), about hindsight combinations of common general knowledge unencumbered by detail which might point the other way. However that point has no relevance to this argument because as a prior art document, Buechler is necessarily not a combination of ideas driven by hindsight knowledge of the invention in issue in this case. Nor does Buechler include or leave out any detail driven by those sorts of hindsight considerations.
800 (Pat), about hindsight combinations of common general knowledge unencumbered by detail which might point the other way. However that point has no relevance to this argument because as a prior art document, Buechler is necessarily not a combination of ideas driven by hindsight knowledge of the invention in issue in this case. Nor does Buechler include or leave out any detail driven by those sorts of hindsight considerations.
349. The second point is a better point at the level of principle. If Buechler does not add significantly to the common general knowledge then evidence addressed to the rhetorical question - if it was obvious why was it not done before? - could be relevant. Illumina contended that there was evidence that those working in the sequencing by synthesis field at the time, before the relevant date, and therefore without knowledge of the invention, were not concerned about photodamage. Illumina based this submission on certain prior published scientific papers. These papers had not been relied on by Prof Greenberg but counsel put them to Prof Johnsson in cross-examination. When I deal with the common general knowledge, it will be convenient to address these papers at the same time. They are not necessarily common general knowledge as such but they illuminate the common general knowledge thinking and motivations of the skilled person and so it makes sense to approach the matter in that way.
350. The relevant common general knowledge is as follows.
351. The wavelength (colour) of a light photon is related to its energy. Shorter wavelength means higher energy and so, since blue light has a shorter wavelength than red light, it follows that blue light photons have higher energy. Fluorescence occurs in the following way. An incoming photon of light is absorbed by the fluorophore molecule. The energy of the photon causes the excitation of an electron from its ground state. The electron is now at a higher energy level. The excited electron may then drop to a somewhat lower energy level by a process called internal conversion. This does not involve the release of another photon. Then the electron may drop back to its original ground state, releasing a new photon. The release of this new photon is the flash of light called fluorescence. The energy of the new photon is lower than and different from the energy of the original incident photon. The corresponding difference in wavelength is called the Stokes shift.
352. Fluorescence happens very fast but once the electron is in the excited state there are other things which might happen instead. One way they can happen is if the excited electron moves to a different set of energy levels called a triplet state by a process called intersystem crossing. That is not quite so fast as fluorescence. One example of what can happen after intersystem crossing is phosphorescence, which is not relevant.
353. It was well known that reactive oxygen species form as fluorophores are put into an excited state by the relevant illumination. These species can include singlet oxygen (which is a highly reactive oxygen molecule O2 in an excited state), hydroxyl radicals, peroxides and superoxide radical anions. There are two main processes called Type I and Type II. The Type I pathway produces free radicals which can react with other things - one effect is to lead to the production of reactive oxygen species such as superoxide, hydroxide and peroxide radicals. Type II only produces singlet oxygen. Singlet oxygen can in turn lead to the production of further reactive oxygen species.
354. The term quantum yield refers to the proportion of incident photons which trigger a given effect. One can have a quantum yield for fluorescence, a triplet state quantum yield, a singlet oxygen quantum yield, and so on.
355. One effect of reactive oxygen species is photobleaching. This refers to damage to the fluorophore molecules themselves. Once reactive oxygen species have damaged the fluorophore it stops being able to fluoresce. The photostability of a fluorescent molecule is its tendency to resist photobleaching, but over time all fluorescent molecules will photobleach.
356. Another effect of reactive oxygen species is photodamage. This is different from photobleaching. The term photodamage is used in this art to refer to the damage to other molecules in the system caused by those reactive oxygen species generated by the fluorophore.
357. As a matter of common general knowledge the skilled person was well aware of both effects. One issue is the extent, in a given set of circumstances, to which a skilled person would regard either or both of these phenomena as a risk worth taking steps to mitigate. I will come back to this below.
358. One well known way to prevent the reactive effects of reactive oxygen species was to use antioxidants. If photobleaching or photodamage was identified as the cause of a problem, it was well known that antioxidants would act to prevent it. Ascorbic acid and ascorbate were well known antioxidants. It is convenient to use the term ascorbate compendiously unless the distinction between ascorbic acid and the salt matters.
359. In another context photodamage was something which was deliberately induced and regarded as beneficial. This was in a field called photodynamic therapy. Here fluorophore compounds were used such as porphyrins and chlorins which generated singlet oxygen species in respectable yields. The purpose of the exercise was to damage biological molecules such as the DNA of cells in cancerous tissue. In this context the fluorophore molecules were called photosensitisers. Certain phthalocyanines were also regarded as efficient photosensitisers.
The scientific papers
Use of antioxidants in papers on sequencing by synthesis
360. The three papers relied on by Illumina are Mitra et al Anal Biochem 320 (2003) pp55-65; Braslavsky et al PNAS April 1, 2003 Vol 100 pp3960-3964 and Seo et al PNAS April 13, 2004 Vol 101 pp5488-5493. These papers came from three well respected sources - respectively Prof Church’s group at Harvard, Prof Quake’s group at Caltech, and the group led by Jingyue Ju at Columbia University.
361. The Mitra (2003) paper is another paper concerning Prof Church’s idea for polonies and sequencing using FISSEQ (a 1999 Mitra & Church paper about the same technique is referred to above in relation to the modified nucleotide patents). To recap, this is a kind of sequencing by synthesis, but it does not use reversible chain terminators. Instead fluorescent labelled nucleotides are added a single kind of nucleotide at a time and then fluorescent detection is used to see if the known kind of nucleotide has incorporated. The technique illuminates fluorophores on labelled DNA primers and on nucleotides.
362. As Illumina points out, the wash buffers disclosed in this paper do not include any oxygen scavengers. One might infer from that that the authors did not perceive a risk of photodamage (or photobleaching) to be sufficient to be worth addressing.
363. Nevertheless the authors did observe a decrease in intensity over time. One of the possible causes of that problem which was identified in the paper itself was “incomplete extension”. Prof Johnsson explained that that would cover incomplete extension due to photodamage. The fact that a later paper showed that the group did not end up finding incomplete extension when they developed the method further does not matter. This leads to another piece of speculation, one might ask whether the absence of an express reference to photodamage in the original paper was because there was in fact no risk in their conditions anyway or was it because there was a risk but they did not realise it? Further, and not a major point, MGI are entitled to note that the original paper did recognise a potential problem, which was something which could have been caused by photodamage, although they did not spell that out and one is again left to speculate.
364. The Braslavsky (2003) paper relates to a single molecule sequencing by synthesis method in which Cy3 labelled nucleotides are used both in their own right and as fluorescence resonance energy transfer (FRET) donors for Cy5 labelled nucleotides. The science is quite involved and the detail is not necessary to understand the points. What matters is that the technique involves multiple rounds of illumination to cause fluorescence and includes a deliberate photobleaching of some of the fluorophores. Photobleaching necessarily involves creating reactive oxygen species, since that is how it occurs. Whether the experiment worked as a sequencing method or not is not really the issue. The important point for Illumina is said to be an absence of concern about photodamage to the DNA.
365. The trouble with Illumina’s case is that the authors did employ an oxygen scavenging system, which in fact included an antioxidant (although that latter point only emerged in closing - which is not MGI’s fault given the way the papers were put without supporting evidence from Prof Greenberg). The authors added the oxygen scavenging system to protect the Cy3 dye which was subjected to repeated illumination. The dye was required to fluoresce on its own or act as a donor in the FRET system, but as I say that detail does not matter. Illumina are entitled to point out that the paper does not say in terms that a concern about photodamage was a reason for the oxygen scavenging system. But as Prof Johnsson pointed out, the oxygen scavenger system would have the effect of reducing photodamage too. Illumina submits that Prof Johnsson agreed it was difficult to infer that the authors were concerned about photodamage. I do not accept that submission (based on one answer in a passage) fairly summarises the professor’s view as a whole. Prof Johnsson knew that he was being asked to speculate about the authors motives. His view was that photobleaching and photodamage go hand in hand, that there was a clear possibility of photodamage occurring and that the system the authors used would act to reduce both. He was agreeing that their prime motivation was to protect against photobleaching, because they were doing that deliberately, but that is as far as his evidence went.
366. The third paper is Seo (2004). This paper is concerned with RCT sequencing by synthesis. It shows five cycles using three fluorescent labelled nucleotides each with a cleavable linker. The linker is photocleavable. The paper was put by counsel to Prof Johnsson on the footing that the authors did not appear to have had any concerns about photodamage. However the professor pointed out to the cross-examiner that they had done the scanning in conditions which scavenged radicals because they used buffers including ethanol. His view was that ethanol was a common general knowledge radical scavenger, and known to reduce photobleaching rates for rhodamines. Nevertheless, as the professor accepted, one again has to speculate about the authors motives. Ethanol might have been used simply as a washing agent (based on a later paper from the Ju Group).
Other scientific papers
367. A number of other scientific papers were also relied on, although they were not about sequencing by synthesis as such. I will not address every reference because it is not necessary to do so, but it is convenient at this stage to address the major ones.
368. Prof Greenberg referred to a paper by Nazarenko (2002) concerning a self-quenching PCR probe. The paper describes multiple cycles of illumination and is another example in which authors might have included an antioxidant had they been concerned about the risk of photodamage to DNA, but appear not to have done, although there is a possible ambiguity, as Prof Johnsson pointed, about stabilizers.
369. Prof Johnsson referred to two papers on single molecule fluorescence: Kapanidis & Weiss and Rothwell. In both of them the authors used ascorbate and other agents to protect against photobleaching. As Illumina are entitled to point out, these papers do not state in terms that a risk of photodamage is a motive.
370. Papers from the field of photodynamic therapy and the use of photosensitisers provide evidence which shows that fluorophores can induce photodamage by generating reactive oxygen species. However I agree with Illumina that such material does not amount to evidence which bears on the assessment of risk by a skilled person thinking about sequencing by synthesis using fluorescently labelled nucleotides at the priority date. That is because for sequencing by synthesis the skilled person would wish to use bright photostable dyes with high quantum yields for fluorescence and low triplet yields. Although photosensitisers would also be desirably photostable, they are also chosen for their high triplet yield and high singlet oxygen yield. Papers mentioned in Prof Johnsson’s report which fall into this class are Boutrine (1996) from Claude Hélène’s group at Inserm and the De Rosa review paper (2002). It is about photosensitisers.
The papers - conclusions
371. Taking the evidence of the three sequencing by synthesis papers in particular, in my judgment they do not support a finding which assists Illumina. The material is not a reliable basis from which one could infer that real teams working in sequencing by synthesis itself did not think photodamage to the DNA was a sufficient risk so as not to take any steps to mitigate it. The Mitra paper is Illumina’s best paper for that submission but it was very early work and it is not clear whether the risk of photodamage in those circumstances has ever turned out to be a material problem anyway in those conditions. The Braslavsky paper does not support any inference in Illumina’s favour. The authors added an oxygen scavenging system for their own reasons. It would have protected against photodamage too. Also the Seo paper does not support Illumina’s case because the authors did in fact use a system which scavenged radicals. Some kind of photobleaching or photodamage may or may not be the reason why they did this.
372. Standing back and considering the many other papers and textbooks in the case, including the ones mentioned above, they refer to conditions different from those in sequencing by synthesis. It was common ground between the experts that one could not infer from results in different conditions what would happen in sequencing by synthesis conditions.
373. The skilled person is well aware that photobleaching and photodamage can occur in theory. The relevant question is what would the skilled person perception of the risk of photodamage to DNA have been if they were embarking on sequencing by synthesis experiments. On this topic the parties were far apart. In summary terms Prof Johnsson’s view was that the risk would be seen as sufficiently high that it was obvious to take steps to mitigate it by adding an antioxidant such as ascorbate, whereas Prof Greenberg did not think the risk was that high and so adding ascorbate was not obvious.
374. Illumina’s submission overall is that the skilled person would choose to use bright and photostable fluorophores in sequencing by synthesis and in that context there was nothing in the common general knowledge to alert the skilled person to the risk of photodamage. That was said to be because bright and photostable fluorophores necessarily reduce the extent to which reactive oxygen species are created and so reduce and risk of photodamage. Prof Johnsson did not accept it was that simple. His view was that the skilled person using a fluorescent system was always aware of that risk. By which he meant an appreciable risk.
375. There was evidence about the intensity of illumination required. One needs to take care with figures expressed in watts because the intensity also depends on the area illuminated and is governed by an inverse square law. No quantitative conclusion can be expressed based on the common general knowledge. Nevertheless the evidence did support a qualitative conclusion. The skilled person at the priority date thinking of using fluorescent detection in a sequencing by synthesis context would regard the illumination likely to be required as relatively intense, albeit applied for short periods. That would have a bearing on their views about risk.
376. Overall on risk, I prefer the evidence of Prof Johnsson. In my judgment, as a matter of common general knowledge, for the fluorescence chemist member of the skilled team, in the relevant circumstances there would always be an appreciable risk of photodamage to other molecules in the system such as DNA. One way of mitigating the risk of photobleaching and photodamage was in the selection of the fluorophore itself but that choice itself bears out the fact that the skilled person regarded these risks as something to take into account and act upon.
The specification of the 412 patent
377. The 412 patent describes the field of the invention as relating to additives for buffers used in fluorescence based multiple cycle nucleic acid sequencing reactions (paragraph [0001]).
378. In the background section at paragraph [0005] the specification explains that when performing sequencing by synthesis using fluorescent labelled nucleotide analogues the brightness of the incorporated fluorophore diminishes at each cycle of nucleotide addition. The inventors found that at around cycle 8 to 10 the sequencing cycles had to stop due to loss of signal. However the inventors also found that if they moved to a different part of the array which had not previously been scanned with the light source used to trigger the fluorescence and detect the nucleotides, the signal was restored, indicating that the problem was being caused by photodamage to the nucleic acid templates. Prof Greenberg’s view was that this was an elegantly designed experiment and one which would not have been obvious to the skilled person.
379. The specification goes on to propose adding ascorbate to the relevant buffer. Results of an experiment comparing the system using a buffer with ascorbate and one without are presented in figure 2. The case with ascorbate is clearly better although there was a disagreement between the experts about how surprising the degree of difference was. I am not persuaded by Prof Greenberg that the skilled person would draw that conclusion from the data presented in the patent.
380. It bears spelling out that the purpose of the buffer is to prevent photodamage to the DNA strand rather than photobleaching to the fluorophores. Every time the fluorophores are illuminated (once per cycle) a fresh lot of reactive oxygen species will be generated which can then go on to react with other molecules. However at every cycle of this process the old fluorophores are washed away and fresh fluorophores are added in the form of fresh labelled nucleotides. Therefore an individual fluorophore molecule does not experience more than one cycle of illumination. However the DNA strand remains in place all the time and so, as the complementary strand grows and the cycles repeat, the DNA will have been present for an increasing number of illumination events, each generating some reactive oxygen species. Note that this explanation follows from what is in the patent, it does not mean that the skilled person, without hindsight, would necessarily see this or think it through in this way. A common slip in patent cases is to equate the fact that the invention can be explained after the event with obviousness. They are not the same.
Claim construction / infringement
381. Claim 1 as proposed to be amended is in this form:
1. A method of sequencing at least two nucleotides of a template nucleic acid by successive cycles of sequencing-by-synthesis comprising repeating the steps of:
a) incorporating one or more fluorescently labelled nucleotides into a strand of nucleic acid complementary to said template nucleic acid; and
b) determining the identity of one or more of the incorporated nucleotide(s), wherein the steps of determining the identity of the incorporated nucleotide(s) is carried out in a buffer which comprises ascorbic acid, or a salt thereof.
[amendment emphasised]
382. Claim 1 claims a method of sequencing at least two nucleotides. As amended it is limited to sequencing by synthesis. That expression includes more than RCT but nothing turns on that here. I will deal with the amendment along with the added matter issue.
383. Step (a) of the method refers to the incorporation of a fluorescently labelled nucleotide into the complementary DNA strand. Step (b) relates to the imaging step. That is the occasion when the ascorbate needs to be in the buffer because that is when the illumination takes place.
384. The inventive concept can be stated as the use of ascorbate during the imaging step to reduce damage to the nucleic acid.
385. In terms of infringement - there is no dispute that MGI uses ascorbate in its relevant buffer. Therefore (save for the E variant) Standard MPS infringes claim 1 and this is admitted. The only issue of infringement of claim 1 relates to Cool MPS. The use of antibodies as the way of labelling the incorporated nucleotide raises an analogous infringement issue to the issue about incorporation which arises for the modified nucleotide patents. Illumina contends there is literal infringement but if not infringement by equivalents.
386. It is convenient to address the infringement point here. Claim 1 of 412 is similar to claim 12 (claim set A) of  578.
578. The method of claim 1 has essentially the same two steps as the first two steps of claim 12, i.e. incorporation of a labelled nucleotide first and determination of the identity of the incorporated nucleotide second. However there is an important difference between the specification of the 412 patent as compared to the modified nucleotide patents in the way incorporation is defined. Unlike the wide definition in the modified nucleotide patents (“becoming part of” a strand), paragraph [0033] of the 412 patent provides a different and narrower definition: “the term ‘incorporation’ of a nucleotide into a nucleic acid strand or polynucleotide refers to the joining of the nucleotide to the free 3’ hydroxyl group of the nucleic acid strand via formation of a phosphodiester linkage with the 5’ phosphate group of the nucleotide.”
The method of claim 1 has essentially the same two steps as the first two steps of claim 12, i.e. incorporation of a labelled nucleotide first and determination of the identity of the incorporated nucleotide second. However there is an important difference between the specification of the 412 patent as compared to the modified nucleotide patents in the way incorporation is defined. Unlike the wide definition in the modified nucleotide patents (“becoming part of” a strand), paragraph [0033] of the 412 patent provides a different and narrower definition: “the term ‘incorporation’ of a nucleotide into a nucleic acid strand or polynucleotide refers to the joining of the nucleotide to the free 3’ hydroxyl group of the nucleic acid strand via formation of a phosphodiester linkage with the 5’ phosphate group of the nucleotide.”
387. This narrower definition focusses specifically on the creation of the phosphodiester linkage. I find that in the 412 patent “incorporation” is limited to a single step and cannot, as a matter of normal construction, encompass a second step which takes place after the phosphodiester bond has been formed.
388. MGI relied on two further points in support of its case on normal construction. I mention them in case this goes further. One was the same kind of point made for the modified nucleotide patents, namely that the examples in the specification are all cases in which the nucleotide acted on by the polymerase has the label already linked to it. In the case on the modified nucleotide patents this did not help. If it were not for the narrow express definition in the 412 patent it would not help here either, but along side that definition it is supportive.
389. The other was the submission that there was said to be no technical reason why the claim would be understood to cover methods where the fluorophore was added after the nucleotide was incorporated. Prof Greenberg’s view was that how the labelling was achieved was immaterial to the second (detection) step (which he called imaging). MGI contended (closing para 189) that the skilled person would understand that there were technical reasons why labelling with the fluorophore after incorporation may affect photodamage.
390. In fact the evidence MGI relied on for this was a paragraph in a confidential annex to Prof Johnsson’s third report in which he explained that there were various potential benefits from doing the labelling using an antibody, as in Cool MPS, rather than having the fluorophore linked covalently to the nucleotide. It does not matter what the particular potential benefits are and since it was in a confidential bit of the report I will not spell them out save to say that one was that the risk of photodamage might be reduced. This evidence does not support MGI’s case on normal construction. In my judgment Prof Greenberg’s evidence referred to above represents the thinking of the skilled person reading the patent. It would support a wide meaning, like the one in the modified nucleotide patents, were it not for the expressly narrower definition in the specification of 412. [After the draft judgment was circulated I was told that MGI no longer maintained the confidentiality of the annex.]
391. Therefore I find there is no infringement on a normal construction.
392. Turning to equivalence, as with the modified nucleotide patents, it is common ground that the first two Actavis questions are answered in Illumina’s favour. The issue is the third question.
393. MGI is right that all the examples in the 412 patent have the nucleotides directly linked to a fluorophore and the specification does not mention the idea of an alternative based on subsequent labelling in general nor the use of antibodies to do that in particular. However these are not strong points in MGI’s favour on the third question.
394. MGI made the same point here which it did for the modified nucleotide patents, that if the idea underlying Cool MPS (using antibody labelling) had been suggested then the skilled person would think it was difficult to raise antibodies. I have preferred Prof Leadlay’s view to the contrary over Prof Johnsson’s view on that score. In the 412 case MGI also referred to what was similar evidence from Prof Marx, that to make it work would require significant research. The fact that MGI called similar evidence from two experts does not improve it. I do not doubt that the work would be significant but I still prefer Prof Leadlay’s evidence. In my judgment the team would be confident it could be done. Therefore this line of thinking does not assist MGI on the third Actavis question.
395. Finally MGI repeated the point that the antibody method “could affect” the extent of photodamage. As explained already, this evidence (in an annex to Johnsson 3) is in fact that the method potentially could reduce photodamage. That is not a reason which supports MGI’s case on equivalents at all. I remind myself that this issue arises because MGI’s Cool MPS antibody method contains ascorbate in the buffer. Using that additive, to reduce the risk of photodamage, is the essence of the invention of the 412 patent and claimed in claim 1.
396. Despite these unconvincing arguments from MGI, in fact I believe the answer to the third Actavis question should be in MGI’s favour for a much simpler reason. The reason why skilled person would think that strict compliance with the normal construction of “incorporation” was essential is because the specification has gone out of its way to define that term in a clear and simple way. It is not necessary for the skilled person to speculate about why the patentee may have done that, the fact is that it has been done. The Cool MPS system is in fact an immaterial variant to the invention claimed in claim 1, but the patent is deliberately drafted in such a way as to exclude it.
397. Claim 15 is in this form:
A kit for use in a method according to any one of claims 1 to 14 comprising:
one or more fluorescently labelled nucleotides, wherein the fluorescent label is linked to the nucleotides via a cleavable linker;
an enzyme capable of catalysing incorporation of said nucleotides into a nucleic acid strand complementary to a nucleic acid template to be sequenced;
and a buffer comprising ascorbic acid or a salt thereof, or a supply of ascorbic acid or a salt thereof.
398. There is no dispute that (again save for the E variant) Standard MPS infringes this claim, and this is admitted. Illumina does not contend that CoolMPS infringes this claim either literally or by the doctrine of equivalents.
Obviousness
399. The skilled person and the common general knowledge have been addressed above, the claims have been construed and the inventive concept identified. If claim 1 is obvious then no other claim would survive. Applying Pozzoli, the next step is to examine the prior art and identify the differences.
Buechler
400. Buechler is a US Patent published in April 2003 entitled “Compositions and methods for inhibiting light-induced inactivation of biological reagents”. It is concerned with the stabilisation of biological reagents conjugated to fluorescent molecules. Buechler states that a problem had been encountered with the stability of these conjugates such that the fluorescent signal would decrease with time; and it was presumed that this was caused by uncoupling of the fluorescent molecule from the reagent but the mechanism was not known. Buechler reports that “for the first time” the mechanism of this degradation has been identified. It is photodamage to the conjugates caused by reactive oxygen species such as singlet oxygen and free radicals. The solution is to use an “oxygen depleting system”. One of the agents mentioned is ascorbate.
401. Nucleic acids are mentioned in Buechler as one of the biological reagents to which this applies but the examples given all use antibodies.
402. All the examples are designed to investigate the activity of antibodies over time under certain conditions (e.g. dark or ambient light) in proximity to fluorophores. Prof. Greenberg’s view was that it was difficult to draw firm conclusions from them because of flaws in the methodology, including the changing of multiple variables at once. The major point is that experiments use various approaches, individually and together, to deplete oxygen. They include argon purging, adding a glucose oxidase system and adding ascorbate. It can be said that there is never a test with results presented which nails down ascorbate alone as an effective agent in those conditions. That is true up to a point but in my judgment the skilled person would not seek to draw conclusions from that experimental data in that way. The key thing is that the skilled person would see, from Buechler as a whole, that the authors had identified that the cause of the problem Buchler was concerned about was photodamage caused by reactive oxygen species which were produced by the fluorescent molecules responding to light. The skilled person did not need Buechler to tell them that if that was the problem then one obvious approach to addressing it was to add ascorbate.
403. For what it is worth Buechler does at one point refer to experiments which showed that ascorbate alone protected against light induced inactivation (col 17 ln65-67) but with the rubric “data not shown”. Given the common general knowledge, I doubt the skilled person would be troubled by the absence of data there. They would have no difficulty believing what Buechler says.
404. However of more significance is the point emphasised by Illumina about the conditions (time and illumination) that Buechler is focussed on. The experiments are mostly focussed on storage of material over periods of hours or days, comparing the effect of storage in the dark with storage in ambient room light. The one example which does not involve storage is example 10 but again here the test is between the assay conducted in the dark (dim green light) against the assay conducted under white room light and the illumination being examined was ambient room light.
405. Illumina also pointed out that Buechler (mostly) used phthalocyanins as the fluorophore molecules. Illumina emphasised the point that some phthalocyanins are used for their appreciable yield of singlet oxygen in photodynamic therapy. I do not accept that this represents a reason why the skilled person who was thinking about a particular assay (in this case some kind of sequencing by synthesis assay) would discount what is described in Buechler as something of no relevance. They would see Buechler as making a general point, supported by experiments in specific circumstances.
406. In terms of differences, turning to claim 1, although the sequencing method claimed is within the wide generality of Buechler (which refers e.g. fluorescent nucleic acid hybridisation is referred to at col 2 ln32-43), Buechler does not disclose the specific sequencing method claimed. Buechler does disclose adding ascorbate alone or with other agents. Claim 1 is not limited to ascorbate alone, it would include using two additives as long as one of them was ascorbate.
Is claim 1 obvious?
407. I start with the reaction of a skilled person, imbued with the common general knowledge of sequencing by synthesis at the priority date, and reading Buechler. The skilled person would not think Buchler added much to the common general knowledge in a broad sense, but it does focus on the occurrence of photodamage caused by conjugated fluorophores in the context of bio assays. A skilled person who read it would be reminded of their common general knowledge that photodamage to other molecules present is something that can occur when fluorophores are used. It would also serve as a reminder that ascorbate, among other expedients, was a possible solution.
408. What would the skilled person think having read Buechler? I do not accept that much is gained for Illumina by the difference in conditions. The skilled person would not approach matters on the basis of trying to reason out relative risk by comparing the conditions used in the experiments in Buechler with those in a sequencing by synthesis experiment. It is manifest that the conditions are quite different in terms of time and illumination intensity. That is not how the skilled person would think. What the skilled person would see in Buechler was a teaching that in the context of bioassays using fluorescent conjugates, there was photodamage to biological molecules in an in vitro context. That included a risk of damage to nucleic acids. They would understand the mechanism which caused this - that illumination of a fluorophore can generate reactive oxygen species which in turn can cause photodamage to other molecules like DNA.
409. MGI submitted it would reinforce the skilled person’s common general knowledge of the risk of photodamage. I agree. That is how it would strike them.
410. An important point, emphasised by Prof Johnsson, is that ascorbate was a stable, soluble and readily available additive which the skilled person would be familiar with and would have no concern about any negative downstream effects. This materially assists MGI’s case. Since, as they were, the mitigation steps were very well known and have no serious drawbacks associated with them, this case is not about a problem where the solution requires any effort to find, is hard to do or comes with significant downsides. The skilled person would not add an additive for no reason, but if they thought there was any appreciable risk that reactive oxygen species might have a relevant effect in this particular context, it would be obvious to add ascorbate.
411. Illumina submitted that the skilled team would use bright and photostable dyes, but as Prof Greenberg agreed, the reason for doing this was to minimise the generation of reactive oxygen species that could bleach the dye and damage the DNA. The professor accepted that having a photostable dye and using a stabiliser like ascorbate were both ways of getting to the same end, which was to minimise photobleaching and photodamage.
412. Standing back, in my judgment the invention is obvious to a skilled person in the light of Buechler. The skilled person would know there was an appreciable risk of photodamage to the DNA given that it will be in the presence of fluorophores and repeated, relatively intense, illumination. One way in which a skilled person could mitigate that risk to some extent would be to select a dye regarded as very bright and photostable but it was equally obvious to choose a moderately bright and photostable dye. Either way it would be obvious to add ascorbate to the relevant buffers in a sequencing by synthesis reaction. The skilled person would have no doubt that whatever conditions and materials were chosen, some reactive oxygen species would be generated. Ascorbate is a well known antioxidant, known to mitigate the inevitable risk.
Alternative cases
413. An alternative case which Illumina perceived MGI to be advancing was that it was obvious to add ascorbate to mitigate a risk of photobleaching of the dye itself. I did not detect such a case but if it was suggested I would not accept it. A skilled person thinking about that would appreciate that any given set of dye molecules only experience one round of illumination.
414. An alternative case which MGI did advance was the submission that even if the skilled person did not think of adding ascorbate at the start, when they ran their experiments they would encounter a fading of the signal in successive cycles (just as reported in the patent at paragraph [0005]). It would be obvious that the cause was photobleaching of the DNA and obvious to apply Buechler at that point and thereby add an antioxidant such as ascorbate. This was Prof Johnsson’s evidence and MGI submitted that it was not challenged in cross-examination.
415. First as a matter of principle, this kind of argument is open to MGI. The fact that it works in a notional way in that experiments which take some time to set up and run before the problem is noticed are treated as taking place at the priority date, is not a valid objection to it (see Merck v Teva [2011] EWCA Civ 382 paragraphs 36-37).
416. Prof Greenberg’s view on this was that four possible causes of the fading would be identified: hybridisation problems, reaction by-products damaging the DNA template, photodamage, and cleavage of the DNA templates from the solid supports. In my judgment this is an example in which the presence of four options does not make any one of them less obvious. For the fluorescence chemist member of the skilled team, the obvious one to look at would be photodamage. Moreover even without doing the experiment described in paragraph [00005] of the patent, which Prof Greenberg had characterised as elegant and non-obvious, the simple and obvious test for the skilled person, especially for a skilled person having read Buechler, would be to add ascorbate. It would tell them whether photodamage was the cause. Prof Greenberg accepted that.
417. I find that even if it was not obvious to decide to add ascorbate to the buffers before carrying out any sequencing by synthesis tests in the first place, the skilled person who took that approach would, acting without any inventive step, encounter a problem of fading and would, without invention, find that it was caused by photodamage by adding ascorbate. The claims are obvious based on MGI’s alternative case too.
Added matter and amendment
418. The law on added matter was set out above in the relevant section for the modified nucleotide patents. The added matter argument here is an intermediate generalisation point directed to something missing from claim 1 as granted (and dependent kit claim 15). Claim 1 is set out above. What is absent from it is essentially any reference to the character of the illumination needed to cause the label to fluoresce (such as being intense). The allegation is that the application as filed (published as WO 2006/064199) only discloses the method of claim 1 together with the requirement that the detection step includes repeated or prolonged exposure to intense illumination. MGI refer to p3 ln3-8 and ln12-18 of the specification in the application and also to claim 1 as filed (at p50 ln3-8). As a matter of the words in the document, MGI is correct. The argument then goes that the difference between the disclosure in the application and the claim as granted is not trivial because although in sequencing by synthesis one generally would use intense illumination, Prof Johnsson explained that that is not necessarily the case. The precise intensity required will depend on the equipment used and the time of illumination and, he said, “I do not think that intense light would strictly be necessary”. Thus argues MGI the application discloses the invention as applicable to a sub-set of sequencing by synthesis processes, namely those which include exposure to intense illumination, whereas the teaching of the granted patent is that the ascorbate containing buffer can be applied to all sequencing by synthesis processes. This is said to be new information and added matter.
419. One point to get out of the way now is the proposed amendment to claim 1. It was shown in the italicised words when the claim was set out above. However if the point is a good one, the amendment will not help. If the argument had been focussed on the absence of the reference to repetitive exposure than the amendment might have achieved something but that is not the issue. Therefore I will not make the amendment.
420. Is MGI right? In my judgment the answer is no. The reason why not is because no new information is provided in the granted patent as compared to what was disclosed to the skilled person imbued with the common general knowledge by the application as filed.
421. I am not persuaded that the skilled person would read the references to intense illumination in the application as filed as a disclosure that the invention was directed to a sub-set of sequencing by synthesis processes. Rather, if they thought about it at all, they would read it as reflective of a shared assumption made by the reader and the inventors that sequencing by synthesis did involve illumination which they would regard as relatively intense. Or putting it another way, while I have no difficulty with Prof Johnsson’s view that the degree of intensity of illumination will vary, I am not convinced that his evidence can be taken to mean that the skilled reader of the application as filed would ever have in mind an idea that there is such a thing as sequencing by synthesis using illumination which would not be fairly regarded as “intense”, particularly given the qualitative nature of the expression itself. I accept Prof Greenberg’s view that the skilled person would understand the reference to “intense illumination” to be a description of the type of illumination they would expect to be required to achieve fluorescence imaging in sequencing by synthesis.
422. Moreover I am not persuaded that the skilled person reading the granted patent as a whole (including the claim) would detect any suggestion that the invention was being taught as applicable not only to an “intense” sub-class of sequencing by synthesis techniques but also to a “non-intense” subclass of sequencing by synthesis too. The teaching about intense illumination in the specification of the application is still present in the granted patent, at paragraphs [0007] and [0008]. The fact the expression is not in claim 1 (or claim 15) would not lead the reader to reason that therefore there is a positive teaching that the invention could be used in other circumstances. If a truly non-intensely illuminated form of sequencing by synthesis was part of the common general knowledge at the relevant time then I suppose the argument might be more tenable, but there was no such evidence.
423. I reject the added matter case.
EP 2 021 415 - labelled nucleotide
424. The skilled person relating to the 415 patent is essentially the same skilled team applicable to the other patents in this case, i.e. a molecular biologist, an organic chemist and a fluorescence chemist. The priority date is 18th May 2006. By this time the idea of sequencing by synthesis was part of the common general knowledge of such a team, as a whole. The fluorescence chemist would receive information about it from the other members.
The common general knowledge
425. The common general knowledge includes various classes of fluorescent dyes. Relevant classes include the xanthenes, cyanines and coumarins. A xanthene group is a three ringed structure. A very well known member of the xanthene class is fluorescein:
426. The triple ringed xanthene structure is at the top. It is the delocalised electron system of this triple ring which makes the molecule fluorescent. The term fluorophore is used to refer to a fluorescent molecule.
427. The fluorescein molecule also has a carboxy group attached to the carbon at position 3 of the benzyl ring which is perpendicular to the xanthene core. This is the ortho-carboxylate. A well known drawback of fluorescein was that in acid and neutral pH a non-fluorescent cyclic lactone would form, known as a spirolactone:
428. Rhodamines are a sub-class of xanthenes in which amino groups are added to the xanthene core. Like fluorescein, the rhodamines also included a substituted benzyl ring perpendicular to the xanthene core. A well known rhodamine was tetramethyl rhodamine:
429. There were a number of commercially available rhodamines available at the priority date. Rhodamines were known to be useful for biological applications because they were more photostable than fluorescein, their absorbance and emission spectra were not affected by changes in pH and most of them exist in an open non-lactone form at physiological pH.
430. The common general knowledge included the idea of functionalising a dye molecule in order to conjugate it to a biological molecule. Common functional groups are carboxyl groups and amino groups. They join together to form an amide bond. When the target biomolecule had one of them it was well known to put the other on the dye to create the amide. The ortho-carboxy group in rhodamines was used for this purpose. One way of activating it was to use N-hydroxysuccinimide (NHS) to form a reactive group called an NHS ester which would react readily with an amine. Commercial dyes would often be available in a variety of different activated or functionalised forms to allow for conjugation to different biomolecules.
431. Linkers were a well known tool to use to connect a fluorophore to a biomolecule and help to prevent interaction between the two. A linker needs a functional group at each end to allow the two molecules to be joined. It should be stable under the relevant conditions and should not fluoresce. There was a modest dispute about how long linkers would be as between Prof Johnson and Prof Greenberg. I find that as a matter of common general knowledge, a linker of 3-6 carbons long was common but other lengths were also common general knowledge.
432. It was well known that just as fluorescein and rhodamines could form a non-fluorescent spirolactone, so also a non-flourescent spirolactam could be formed if the ortho-carboxylate was reacted with an amine to form an amide. The way to avoid it, as the skilled person knew, was to use a secondary amine to react with the ortho-carboxy group and not a primary amine. With a secondary amine, the resulting amide is a tertiary amide, which does not form the spirolactam (to any relevant extent) in relevant conditions such as pH 7. Whereas if a primary amine had been used, the result would be a secondary amide, which will form the spirolactam at pH 7. This is illustrated in the diagram below:

433. In terms of selecting fluorophores for labelling biological molecules, the skilled person would select a fluorophore with spectral properties suitable for their equipment and suitable to allow unambiguous detection. So if four fluorophores were to be used to sequence DNA, they would need to be distinguishable. The fluorophores should be bright and photostable and not interfere with the reactions taking place in sequencing by synthesis. In reactions taking place in water, aqueous solubility was an advantage.
434. An important issue is the effect which the exercise of connecting a fluorophore to a biomolecule could have on the photochemical properties of fluorophores. Those properties include the absorbance and emission wavelengths, quantum yield and extinction coefficient, and more generally the brightness, photostability and tendency to quench. In the end the evidence was clear. The skilled person knew that the spectral properties of xanthene based dyes can be altered by changes or substitutions on the xanthene core. That is because they affect the delocalised system in that core. So a linker which affected the delocalised system in the xanthene core may affect the photochemical properties of the dye. The skilled person also knew that changes made further away from that xanthene core were less likely to make any change to the spectral properties of the system while changes closer to the core were more likely to have an effect.
435. However in the end this is an empirical field. The characteristics of individual moieties within a chemical structure are influenced by their environment. Molecules can be drawn out flat on a page but as the skilled person knows they are in fact three dimensional structures which can move. Parts which look remote on the page can interact with more distant parts because the whole molecule can adopt a shape in which that is true. Given a known fluorescent molecule and a new molecule derived from that known fluorescent molecule e.g. by adding a linker of some kind to it, and given the photochemical properties of both forms, the skilled person can rationalise whatever differences do or do not exist in the photochemical properties by reference to those structural changes. However that is not the same as being able to predict in advance with any degree of precision what the effect of a given change would be.
The patent and claim construction
436. The 415 patent is directed to what it calls novel rhodamine dye compounds, as well as conjugate molecules comprising those dyes used to label nucleotides, and methods for the use of these conjugates in sequencing by synthesis (paragraph [0001]). A general formula for the rhodamine dye compounds is in paragraph [0007]. The patent refers in general terms to a labelled nucleotide conjugate as being defined by the formula “N-L-Dye”. In this formula N is the nucleotide, L is a linker (and Dye is the dye).
437. The specification goes on to disclose a number of dyes, one of which is called Dye 2 in Example 1 at paragraph [0084]. Dye 2 has the following structure:

438. The patent then explains how to conjugate its rhodamine dye compounds to nucleotides. To facilitate this, the first step is to derivatise the dye by adding a linker arm to the ortho-carboxy group. One example given in the 415 patent is a carboxy-functional linker arm shown below:

439. The carboxy group is at the end of the linker arm on the upper right hand side of the molecule as drawn. This functional group facilitates the conjugation to something else such as a further linker. Whether the further linker and the linker arm are regarded as one linker or two (or the linker arm is seen as part of the dye) is something I will come back to.
440. The 415 patent then discloses some linkers that can be used for covalent attachment of the dye to a nucleotide, via the linker arm above. One linker is called LN3 in Example 4. It has the following structure:

441. Note the azide group roughly in the middle. It makes the linker cleavable.
442. In Example 5 the 415 patent describes the creation of a conjugate molecule which involves connecting together a derivatised dye (Dye 2 + linker arm), the linker (LN 3) and a 3' azido-methyl blocked nucleotide tri-phosphate. The nucleotide is thymidine. I will refer to the resulting molecule as the Example 5 compound. It is:

443. The 415 patent then discloses the synthesis of three other 3' azido-methyl blocked nucleotide tri-phosphate molecules, each with a different fluorescent dye (Examples 6, 8 and 9). The result is a suite of four dye labelled conjugate molecules corresponding to nucleotides A, C, G and T, each of which is labelled with a different dye so that it might be separately identified by fluorescence.
444. A point arises on example 9. It relates to the base guanine. In this example the link between the dye (in this case Atto 532 NHS ester) and the nucleotide has an extra aspect. There is an 11-mer run of polyethylene glycol (PEG). This spaces the dye further away from the nucleotide. As the 415 patent explains, this is used to increase the fluorescent intensity. The reason is because guanine is known to have a tendency to quench fluorophores.
445. Example 11 then discloses the use of those four labelled modified nucleotides in a sequencing reaction of a DNA template of known sequence using fluorescent detection by a repeated cycle of incorporation and cleavage. In other words it discloses a method of sequencing by synthesis using reversible chain terminators. The scanning is done with four colours (paragraph [0194]). Paragraph [0203] of the 415 patent explains that during the first 20 cycles, the error rate was less than 1%.
446. This indicates that the SBS system performed well and that the fluorescently labelled modified nucleotides produced a clear signal to permit reliable detection.
447. The only relevant claim in this patent is claim 1 in the form proposed to be amended unconditionally, which is claim 3 as granted. The claim is in this form:
A nucleotide labelled with a compound according to the formula:
448. This is therefore a product claim to a nucleotide labelled with the compound made from derivatised Dye 2 (including the linker arm) and linker LN3. The claim covers a derivatised form of an 3' azidomethyl blocked nucleotide tri-phosphate molecule labelled with the compound shown. The amendment is not opposed and I will make the relevant order.
449. There are three amide linkages between the two benzyl rings in the upper part of the formula shown in the claim. I will refer to the middle amide of the three as the middle amide of claim 1.
450. The claim is narrow in scope in that the dye moiety and the linker moiety are specified, nevertheless it is not a claim to a single molecule, because it is not limited to any particular nucleotide. The term nucleotide in the claim will include derivatised nucleotides able to be conjugated to the molecule shown in the formula of the claim and will include 3' blocked forms of that nucleotide. Nevertheless it is convenient for the purposes of discussion to refer to this claim as if it related to a single compound. Nothing turns on the fact that the claim in fact covers compounds with different nucleotides.
451. There is no issue about infringement. MGI accepts that it has made, used and sold a compound within what is now claim 1 in its RCT sequencing systems in issue in this case.
452. The issue is validity over Milton and Arnost and an insufficiency squeeze. Milton is a patent from the Solexa group. Essentially MGI’s case is as follows. Milton discloses the idea of using labelled nucleotides with cleavable detectable fluorescent labels in a sequencing by synthesis process. In particular it discloses a nucleotide labelled with a fluorescent dye called Cy3 which has been conjugated to the nucleotide using a linker which is in fact linker LN3 in the 415 patent and is part of the molecule claimed in the 415 patent. Milton teaches that this is a useful product in sequencing by synthesis. On the other hand Arnost (a patent from the Polaroid company published in 1990) discloses fluorescent dyes for making in labelled conjugates for use in biological diagnostic assays. Compound XVI of Arnost is in fact Dye 2 of the 415 patent. Indeed Arnost teaches derivatisation of the dye moieties, albeit the linker arm used in the 415 patent is not disclosed.
453. MGI then argues that in the light of this prior art claim 1 is a mere collocation along the lines of Sabaf v MFI [2004] UKHL 45 and as recognised in some European Patent Office decisions and in the EPO Guidelines for Examination. MGI argues that in the claimed molecule the nucleotide, the linker and the dye each act independently of one another. They each perform the function for which they were already known in the art (or was obvious). However the whole is not greater than the sum of the parts. There is no synergy in this combination. And so no inventive step.
454. Importantly MGI does not say, and has no evidence that, it would have been obvious for a skilled person given Milton to use the dye XVI disclosed in Arnost. One can well understand why such a case would not have succeeded. It is hard to imagine, absent hindsight, that the skilled person would ever alight on dye XVI of Arnost having read Milton. There is no evidence that the application of the common general knowledge by a skilled person reading Milton would lead to the Arnost document at all. Notably dye XVI of Arnost (aka Dye 2 of the 415 patent) was not a commercially available rhodamine dye at the priority date.
455. MGI’s submission is that it does not have to prove that it would have been obvious for a skilled person given Milton to use the dye disclosed in Arnost. The reason why not is because the combination of the nucleotide plus linker of Milton with the dye of Arnost has no extra technical effect or benefit, over and above the known effects of those components acting independently.
456. As an alternative MGI argues that the claim makes no technical contribution to the art and is invalid as Agrevo obvious for that reason. The Milton prior art teaches that a fluorescent dye labelled nucleotide with this linker will be useful in sequencing by synthesis, and no further contribution to the art is made by the 415 patent showing that a particular dye used with that linker/nucleotide combination is useful in sequencing by synthesis.
457. Illumina does not agree. Its case is that the compound of claim 1 is a useful compound, that its utility derives from the compound as a whole and cannot be parsed down to be nothing more than the sum of the parts. Therefore the collocation argument fails. Illumina also submits that the successful use of the compound taught in the 415 patent’s 20 cycle sequencing reaction with a 1% error rate represents a technical advance over Milton and an answer to MGI’s alternative case.
458. Illumina also points out that despite the researches of its legal team, there is no case in which the collocation principle has been applied to a case about a chemical molecule. The submission is that this is not an accident. It is because the argument cannot succeed when applied to that sort of invention.
459. To address this I will start with the law, then deal with the common general knowledge, resolving the relatively small number of points on the evidence of Prof Greenberg and Prof Johnsson, and then address the grounds of invalidity.
The law
460. I do not need to go over the law on lack of technical contribution, the point of law to be addressed concerns collocation.
461. The leading case is Sabaf. Lord Hoffmann gave the single judgment. At trial Laddie J had found the claim was invalid, applying the “law of collocation”, much along the lines argued in the present case by MGI. Laddie J made reference to Lord Tomlin in British Celanese v Courtaulds (1935) 52 RPC 171 and to the EPO Guidelines. The Court of Appeal overturned that decision because they found the law to be that it was impermissible to combine two prior art disclosures unless it was obvious to combine them. Lord Hoffmann held that the Court of Appeal’s approach was contrary to both English and EPO law (paragraphs 19-24). He summarised the applicable principles in two ways. First in paragraph 24 he approached the matter on the basis that there may be two inventions in a single patent claim, holding that:
“24. […] I quite agree that there is no law of collocation in the sense of a qualification of, or gloss upon, or exception to, the test for obviousness stated in s.3 of the Act. But before you can apply s.3 and ask whether the invention involves an inventive step, you first have to decide what the invention is. In particular, you have to decide whether you are dealing with one invention or two or more inventions. Two inventions do not become one invention because they are included in the same hardware. A compact motor car may contain many inventions, each operating independently of each other but all designed to contribute to the overall goal of having a compact car. That does not make the car a single invention.”
462. Then, after dealing with s14 of the 1977 Act, Lord Hoffmann said this:
“26. The EPO guidelines say that “the invention claimed must normally be considered as a whole”. But equally, one must not try to consider as a whole what are in fact two separate inventions. What the Guidelines do is to state the principle upon which you decide whether you are dealing with a single invention or not. If the two integers interact upon each other, if there is synergy between them, they constitute a single invention having a combined effect and one applies s.3 to the idea of combining them. If each integer “performs its own proper function independently of any of the others”, then each is for the purposes of s.3 a separate invention and it has to be applied to each one separately. […]”
463. This is the paragraph of Lord Hoffmann’s judgment which explains the principles to be applied in identifying a collocation case. In the passage he merged together phrases from Lord Tomlin and from the EPO Guidelines for Examination.
464. The current version of the EPO Guidelines contains the same passage as was cited in Sabaf. It is fair to say that collocation cases are not common, but that does not undermine the importance of the principle itself.
465. Illumina drew attention to a passage in the extract from Lord Tomlin’s speech in British Celanese which is cited in Sabaf. Lord Tomlin’s way of characterising the first case I have mentioned above was to as one in which the prior art features, when placed together, “have some working inter-relation producing a new or improved result”. MGI suggested this was wrong in the light of Sabaf. I do not believe it is, provided it is understood in the context of what Lord Hoffmann said. The new or improved result has to be the result of the relationship between the parts of the combination.
466. Although the parties did not refer to it, I derive two further points relating to this from Kitchin J’s judgment in Abbott Laboratories v Evysio [2008]  EWHC
EWHC 800 (Pat) at para 182-185.
800 (Pat) at para 182-185.
467. In paragraph 182 Kitchin J said that the first step (in considering a case like this) is to determine whether the claim is concerned with a single invention or not. The significance I derive from that is a reminder that the applicability of this principle is decided by reference to the claim, rather than with a focus on what is obvious from the prior art (which at least appeared to be how the arguments before me were mostly focussed).
468. In paragraph 185 in expressing his conclusion that the claim in that case was not simply a collocation of elements which perform their own functions independently of each other, Kitchin J referred to the fact that there was an interaction between the elements which the designer of the relevant product must take into consideration. Therefore each element cannot be regarded as an individual invention for obviousness purposes. I agree that this is a material consideration.
469. The principle is a general one and so, as a matter of law, it is capable of applying to chemical molecules as much as to anything else, at least in theory.
Inventive step
470. The skilled person and the common general knowledge have been addressed. I will address the prior art first, because if the elements are not obvious at all then the collocation issue does not arise.
Milton
471. I agree with MGI that Milton discloses the idea of using labelled nucleotides with cleavable detectable fluorescent labels in a sequencing by synthesis process. The idea of using 3' blocked bases A G C and T in an RCT form of sequencing by synthesis is disclosed (see the passage from p1 - p3 and in particular at p2 ln 8-10). The essential idea disclosed is to conjugate a suitable fluorophore, via a suitable cleavable linker, to a suitably functionalised nucleotide. A suitable functionalised nucleotide is shown at p38.
472. At p18 Milton explains that the method can be used with conventional detectable labels such as fluorophores. Dyes Cy3 and Cy 5 are mentioned. The passage also proposes that other commercially available fluorescent labels include, but are not limited to fluorescein, rhodamine, and a number of other classes of dye such as coumarin.
473. There is a point on p21 of Milton. Here the document explains that the linker can contain a spacer unit which distances the nucleotide base from the cleavage site in the linker or from the label attached to the linker. The teaching in this passage from ln11-20 is about avoiding interference with the polymerase reaction. The length of the linker is unimportant as long as the label (i.e. the fluorophore) is held a sufficient distance from the nucleotide to avoid that interference.
474. One linker is disclosed at p47-48. It is the same molecule as LN3 in the 415 patent. The linker is attached to a Cy3 fluorescent dye at page 49. The resulting molecule is the following:
475. The linker (which is LN3) is shown on the upper right hand side. Dye Cy3 was presented in a commercial available mono NHS ester form. The amide bond between the linker and the derivatised dye can be seen left of centre in the lower molecule. Cy 3 is the part on the farthest left. At page 50 Milton then shows this linked dye conjugated to a derivatised nucleotide to give a labelled nucleotide:
476. Milton explains that the linked dye and the whole dye-linker-nucleotide conjugate were each quantified by measuring their absorbance at 550 nm, indicating to the skilled reader that these changes did not affect this aspect of the photochemical properties of Cy3.
477. A thymidine form of the same labelled nucleotide is then used in two cycles of sequencing by synthesis from p52 of Milton. The results are shown in the form of gels using radiolabelled 32P. No results are presented based on using fluorescent detection. The gels show that the linker did not prevent incorporation of the nucleotide, although there is evidence in the gels that incorporation was not 100%. Incorporation at the second cycle is reasonably clear. The skilled person would see these results as supportive of the teaching of Milton that the linker should not interfere with the polymerase reaction.
478. In terms of claim 1 of the 415 patent, Milton discloses a nucleotide labelled with a molecule identical to the claim up to the middle amide of claim 1. In other words Milton discloses what I will call a derivatised nucleotide plus linker LN3. It would be obvious for a skilled person, given Milton, to make that linker-nucleotide combination with a view to conjugating that combined molecule to a suitable dye, which may have to be derivatised. They would make this molecule with a reasonable prospect that if they tested it in sequencing by synthesis, it would not interfere with the incorporation reaction catalysed by the DNA polymerase.
479. However, at the risk of repetition, there is no case that it would be obvious for that skilled person in those circumstances to alight on dye XVI of Arnost at all. The reference to rhodamines does not take the skilled person to Arnost on any view. At one stage MGI appeared to suggest that the olanzapine case Dr Reddy’s v Eli Lilly [2009] EWCA Civ 1362 was relevant on the basis that dye XVI could be regarded as a selection from the general class of dyes disclosed in Milton by the word “rhodamine” on p18. That is wrong for two reasons. First, the term rhodamine is not a disclosure of every individual molecule which could be called a rhodamine dye, just as connecting means does not, as a matter of disclosure, disclose a nail. The latter may (or may not) be obvious from the former but that is another matter. Dye XVI is not disclosed by p18 of Milton at all. Second, p18 in fact only refers to commercially available dyes, and there is no evidence dye XVI was one of those at the priority date. Therefore Dr Reddy’s v Eli Lilly is not relevant.
Arnost
480. Arnost relates to fluorescent dyes for use in making in labelled conjugates to be used in biological diagnostic assays. At col 1 ln36 Arnost sets out a number of generally useful properties of fluorophores in this context. They should have relatively long emission wavelengths (above 500nm), a large Stokes shift, be stable in assay conditions, be relatively free of non-specific interference both with the materials in solution and the moiety to which the dye is conjugated, and provide high quantum yields. Arnost proposes some rhodamine dye molecules for this purpose. A general formula of a conjugated molecule is given at the top of col 2. The biologically active moiety in the defined formula is stated in the most general way possible.
481. At col 3 Arnost refers to attaching the dye to the biological moiety by a “divalent achromophoric linking group”. This is a linking group which does not cause appreciable shift in the spectral absorption characteristics of the dye moiety. It is divalent so that one end can be attached to the dye moiety and the other end to the biologically active moiety. A DNA probe is mentioned as one possible biologically active molecule. At col 4 Arnost states that the dye moieties disclosed typically have absorbance maxima between 500-650 nm, Stokes shifts of 15-20 nm and high quantum yields of 0.7-0.8. They also can have solubilising groups attached to improve solubility.
482. At col 5 ln53-col6 ln58 Arnost refers to various bio-assays in general terms. Unsurprisingly given its date, there is no express reference to sequencing by synthesis.
483. Most of Arnost is given over to explanations of the chemical synthesis to produce the relevant molecules. This starts with molecule IX which is a rhodamine dye with methyl ester groups attached to nitrogen atoms substituted into the xanthene core. This dye is linked to the biologically active compound theophylline via a piperazine linker. The resulting compound is XI:
484. Starting molecule IX can be seen on the left, the piperazine linker is in the middle, connected on its left to the ortho-carboxylate of starting compound IX. Theophylline is on the right, connected to the other end of the piperazine linker via a carboxy propyl group. The steps were to create carboxypropyl theophylline first, then add the linker to that and then connect this to dye IX.
485. Most of the examples in Arnost use theophylline as the biologically active molecule but thyroxine and phenobarbital are also mentioned. The examples always use a piperazine linker of one sort or another. Various particular dye molecules are described. In the last example (Example VII) a thyroxine bioassay is described using a fluorescent conjugate. The example does not state which conjugate was used but the reader would infer it was probably molecule XXIII because that one was probably the more soluble of the two molecules described in the thyroxine example IV. The absorbance maxima and extinction coefficients for some of the molecules described are stated.
486. One of the molecules described is molecule XVI. It is in example III. It is:

487. In the example a piperazine linker is added to it to make molecule XVII. Molecule XVIII consists of this dye attached to carboxylpropyl theophylline via the piperazine linker. No photochemical properties for molecules XVI, XVII or XVIII are given in Arnost.
488. The skilled person would infer that the two sulphonate groups attached to the nitrogens in the xanthene core of molecule XVI would improve its solubility. For what it is worth they would also take it that molecule XXIII (which does not have sulphonate groups and which was probably used in example VII) was soluble too.
489. In terms of the patent in suit, of course molecule XVI of Arnost is Dye 2 of the 415 patent. Therefore Arnost discloses a molecule identical with the left hand side of the formula of claim 1 and teaches its use in making conjugates with biologically active molecules. Note however that molecule XVI stops at the ortho-carboxyl group on the benzyl group below the xanthene core. Arnost does not disclose the linker arm of claim 1 which goes from that carboxyl group to the middle amide of claim 1.
490. In terms of obviousness, I am quite sure it was obvious for a skilled person at the priority date concerned with labelling biological molecules, given Arnost, to take molecule XVI forward as a candidate fluorophore. They would assume it had spectral properties in the range taught by Arnost and nothing in their common general knowledge would indicate otherwise. Nevertheless I was not persuaded by MGI’s attempt to say that a comparison with known Atto dyes would make molecule XVI even more obvious but that does not matter.
491. The sulphonated nature of compound XVI would make it attractive because it would be expected to improve its solubility in an aqueous medium.
492. It would be obvious to use a linker of some sort rather than attempting to conjugate the dye directly to the biological molecule. The linker would be, as Arnost states, divalent and achromophoric. An obvious group to use at the end of the linker which was to connect to the dye would be an amine. Based on Prof Johnson’s evidence, I find that it would be obvious to use a secondary amine here to avoid spirolactam formation. A linker 3-6 carbons long would be a sensible choice here, as Prof Greenberg accepted. All options (3, 4, 5 and 6 carbons) are obvious. It would be an obvious option to have a suitable functional group at the other end of this linker in order to link to the next thing. So a carboxyl group would be used to link to an amine group.
493. Accordingly I find that given Arnost, an obvious molecule for the skilled person to make in the context of labelling biologically active molecules in a bioassay, is the left hand end of the formula of claim 1 of the 415 patent up to the middle amide. It is the 4 carbon option. I will call this molecule the 4 carbon linker form of dye XVI.
494. The skilled person would not know whether the photochemical properties of the 4 carbon linker form of dye XVI were identical to those of molecule XVI itself. They might or might not be, but the skilled person would expect any change to be modest but not necessarily trivial.
495. However, there is no case that it would be obvious for that skilled person in those circumstances to alight on the disclosure of Milton in these circumstances or onto a nucleotide conjugated to what is linker LN3 as shown (but not named that way) in Milton.
Lack of technical contribution
496. Although this is MGI’s alternative case, it is convenient to deal with it first. The argument is run over Milton. The question is whether the claimed molecule makes a technical contribution over Milton. The submission is that the specific rhodamine dye claimed in the 415 patent within the molecule of claim 1 does not provide any technical advance over Milton’s teaching to use rhodamines in general. The dye in the claim has no beneficial properties over rhodamines in general. It is an arbitrary dye. These are MGI’s submissions.
497. In my judgment this argument fails (or at best adds nothing to the collocation point) because the 415 patent discloses that the molecule of the claim, including as it does the relevant dye moiety, is a useful compound with beneficial properties. It is an effective sequencing reagent. The beneficial properties are that it can be used in a sequencing by synthesis scheme of the kind described (fluorescent detection, 20 cycles, 1% error rate etc.). The dye fluoresces, the linker can be cleaved, and the nucleotide can be incorporated by the DNA polymerase. Milton’s results did not demonstrate that all this would happen for any given choice of combined molecule. For example, there were not 20 cycles in Milton, nor four nucleotides at once with different dyes, and the detection used radiolabelling not fluorescence. Milton did not show that the Cy3 dye would fluoresce in the conditions required. There is valuable technical information in the 415 patent which was not made available to the public by Milton.
498. It is also important to note that none of this is inconsistent with the findings on sufficiency which were made on the modified nucleotide patents. The fact that there is no undue burden putting the modified nucleotide patents into practice, which would involve making modified nucleotides linked by suitable linkers to suitable fluorophores and running them in multiple cycles, does not mean that a particular molecule which is in fact an effective fluorophore linked nucleotide is not useful. This Agrevo objection is not based on the proposition that it was obvious to come up with this particular molecule or obvious that it would have those properties. To be an effective objection, this objection is that the claimed molecule is not useful at all. But it is.
499. So I reject the attack based on Agrevo and lack of technical contribution as a separate matter. Therefore the obviousness case all turns on collocation.
500. A striking point is that despite the narrow scope of this claim, MGI infringes it. MGI is using the claimed molecule. I have not taken it into account although it might be said to be at least some evidence from which one would be entitled to infer that the molecule is useful. MGI’s counsel in closing made some submissions about how the use of the molecule came to have happened and what MGI were doing about it, but they were not supported by evidence and in any case do not take away from the fact that MGI are using the claimed molecule in their commercial sequencing by synthesis systems.
Milton and Arnost side by side - collocation
501. On the conclusions I have reached, it follows that claim 1 will be invalid if it is a collocation.
502. Claim 1 can be seen as a combination of two aspects:
i) the 4 carbon linker form of dye XVI; and
ii) a derivatised nucleotide plus linker LN3.
503. The place where they join is the middle amide bond. The findings so far mean that each of the two elements of claim 1, taken on its own, is obvious, but it would not be obvious to combine these two elements.
504. MGI point to the fact that the 415 patent is written on the basis that the claimed molecule is made up of building blocks - dye, linker, nucleotide etc. That is true but while it gives some support to the argument, in the end it is not determinative. Almost all inventions can be described as being made up of parts but that is not an admission by the patentee that the parts form a mere collocation.
505. As mentioned already, the claimed molecule as a whole has useful properties (fluorescent detection, 20 cycles, 1% error rate etc.). However just because the combined molecule has useful properties does not answer the question. The hob in Sabaf was useful - it took up as little vertical space as possible. However this useful property was the result of two obvious independent features operating independently, and so it was invalid.
506. In the common general knowledge section I addressed the skilled person’s views thinking about the effect on photochemical properties of connecting a fluorophore to a biomolecule and the degree of predictability in this field.
507. At paragraphs 205 and 206 of his first report, Prof Greenberg addressed whether a dye, linker and nucleotide function independently of one another. His view was that adding a new linker represented a significant change to a fluorophore, creating a new molecule whose physiochemical and photochemical properties may differ from the unsubstituted fluorophore. His view was that the linker and fluorophore would not be considered as two elements in isolation and that due to the number and complexity of all the factors at play, it was (and still is) difficult to predict how, and the extent to which, these properties would be affected. Therefore he believed that the skilled person would consider testing desired fluorophore-linker combinations to be necessary. An example he referred to was a possible effect on solvation.
508. The point of disagreement with Prof Johnsson was about the degree of predictability. His evidence (paragraph 112 of his third report), was that whatever effects were caused were unlikely to have any impact on the photochemical properties of the fluorophore.
509. I was not convinced that things are as predictable for the notional skilled person as Prof Johnsson said, and if a finding is required, I prefer the evidence of Prof Greenberg on this aspect. However in my judgment the debate about predictability here in fact misses the point. Prof Johnsson was not saying that the linker was incapable of having an effect on the dye. His evidence was just that it was unlikely. That would be powerful evidence in an obviousness case in which one of the steps to be considered was what the prospects of success might be in combining in a single molecule the 4 carbon linker form of dye XVI with a derivatised nucleotide plus linker LN3. But that is not the relevant question.
510. As a matter of fact the claimed thing is a single molecule. The evidence is clear that these two aspects of that molecule are capable of interacting with one another. There is a potential for interaction between these aspects which the skilled person must always take into consideration. The fact the interaction would be one which is unhelpful does not mean it is not relevant. Moreover in this, essentially empirical, field the skilled person will not know whether or not there is in fact an interaction until a test is done. In fact the tests are not burdensome, but they would need to be done. In that sense this is a long way from Sabaf because there is no basis in that case for thinking there might be an interaction and then looking to find out. The two aspects in Sabaf simply do not interact with one another. The skilled person did not have to test them to find out. A vice in MGI’s case is that it seeks to mix together considerations about things being obvious to try with the collocation principle.
511. As I have indicated already, unlike Sabaf this case is about unwelcome interactions. The dye fluoresces satisfactorily in the 415 patent because in fact the linker and nucleotide do not interact with it in an unfavourable way. They could have but they do not. The dye/linker combination, which is different from the one tested in Milton, does not in fact prevent incorporation of the nucleotide with the DNA polymerase. It might have but it did not. In this sense the circumstances are quite different from Sabaf. There was no suggestion there that combining the primary air flow from above with a flame spreader using the Venturi effect was even capable of having an unfavourable interaction. The designer in that case would have regarded the two parts as entirely functionally distinct before putting them in one oven.
512. If a single molecule were made consisting of the 4 carbon linker form of dye XVI and a derivatised nucleotide plus linker LN3, the skilled person would believe that these two parts are capable of interacting with one another. There is nothing inherent in either element to mean that it is incapable of interacting with the other element. The dye part is not a priori immune from the effects of the linker or the nucleotide. The skilled person would hope the molecule worked satisfactorily because the two elements did not interact but they would need that to be demonstrated by an experiment testing the combination as a whole. That means that the collocation principle does not apply.
513. To establish that this claim is obvious it would be necessary to show that it was indeed obvious to make the single entire molecule for the purposes of testing it. I can see that if that indeed had been obvious, then the obvious to try test might be satisfied in MGI’s favour, but it was not and the point does not arise.
514. Putting it another way, the molecule of claim 1 is a single invention. Its beneficial properties derive from the functional relationship, which includes non-interference, between the constituent parts. I find that claim 1 of the 415 patent is valid.
Conclusion
515. EP (UK) 1 530  578
578 with claims as amended in the form of claim set A is valid. All four of the MGI systems known as Standard MPS, Cool MPS, the two colour variant and DNBSEQ E infringe each of claims 1, 7, 12, 20, and 24 (claim set A) of that patent.
with claims as amended in the form of claim set A is valid. All four of the MGI systems known as Standard MPS, Cool MPS, the two colour variant and DNBSEQ E infringe each of claims 1, 7, 12, 20, and 24 (claim set A) of that patent.
516. EP (UK) 3 002 289 with claims as amended in the form of claim set B is valid. All four of Standard MPS, Cool MPS, the two colour variant and DNBSEQ E infringe each of claims 1, 5 and 6 of that patent. In relation to claim 4, it is infringed by Standard MPS and Cool MPS but not by the two colour variant or DNBSEQ E. The amendment to claim 9 is allowable but Illumina needs to explain what the amendment is for.
517. EP (UK) 3 587 433 with claims as amended in the form of claim set C is valid. All four of Standard MPS, Cool MPS, the two colour variant and DNBSEQ E infringe each of claims 1 and 6 of that patent.
518. EP (UK) 1 828 412 is invalid because claim 1 is obvious over Buechler. Claim 1 is not invalid for added matter. Standard MPS and the two colour variant fall within claim 1 but since that claim is invalid, they do not infringe. Cool MPS does not fall within claim 1 regardless of validity either on a normal construction or under the doctrine of equivalents.
519. EP (UK) 2 021 415 as amended down to claim 3 as granted is valid. Standard MPS infringes that patent.
520. I do not address the s71 point in this judgment.
Annexes - claim sets of the modified nucleotide patents
Claim set A - Claims of  578
578 as proposed to be amended
as proposed to be amended
[The amendments are shown in green and red. Red shows the changes in the first set of amendments as compared to the granted claims, green shows the further changes. The only conditional amendment is to claim 12 as granted (7 as amended). The rest is unconditional and unopposed.]
1. A modified nucleotide molecule comprising a purine or pyrimidine base and a ribose or deoxyribose sugar moiety having a removable 3’-OH blocking group covalently attached thereto, such that the 3’ carbon atom has attached a group of the structure
-O-Z
wherein Z is any of -C(R’)2-N(R")2’C(R’)2-N(H)R", and -C(R’)2-N3, wherein
each R" is or is part of a removable protecting group; each R’ is independently a hydrogen atom, an alkyl, substituted alkyl, arylalkyl, alkenyl, alkynyl, aryl, heteroaryl, heterocyclic, acyl, cyano, alkoxy, aryloxy, heteroaryloxy or amido group, or a detectable label attached through a linking group; or (R’)2 represents an alkylidene group of formula =C(R’’’)2 wherein each R’’’ may be the same or different and is selected from the group comprising hydrogen and halogen atoms and alkyl groups; and wherein said molecule may be reacted to yield an intermediate in which each R" is exchanged for H, which intermediate dissociates under aqueous conditions to afford a molecule with a free 3’OH an azidomethyl group.
2. A molecule according to claim 1 wherein R’ is an alkyl or substituted alkyl.
3. A molecule according to claim 1 or claim 2 wherein - Z is of formula -C(R’)-N3.
4. A molecule according to any one of claims 1 to 3 wherein Z is an azidomethyl group.
5. A molecule according to claim 1 or claim 2 wherein R" is a benzyl or substituted benzyl group.
62. A molecule according to any preceding claim 1 wherein said base is linked to a detectable label via a cleavable linker or a non-cleavable linker.
73. A molecule according to claim 62 wherein said linker is cleavable.
84. A molecule according to any one of claims 1 to 5 wherein a detectable label is linked to the molecule through the blocking group by a cleavable or non-cleavable linker.
954. A molecule according to any one of claims 62 to 843 wherein said detectable label is a fluorophore.
1065. A molecule according to any one of claims 62 to 954 wherein said linker is acid labile, photolabile or contains a disulfide linkage.
1176. A modified nucleotide molecule as claimed in any one of claims 1 to 1065 which comprises one or more 32P atoms in its phosphate portion.
1287. A method of controlling the incorporation of a nucleotide as defined in any one of claims 62 to 1065 and complementary to a second nucleotide in a target single- stranded polynucleotide in a synthesis or sequencing reaction comprising incorporating into the growing complementary polynucleotide said nucleotide, the incorporation of said nucleotide preventing or blocking introduction of subsequent nucleoside or nucleotide molecules into said growing complementary polynucleotide.
1398. The method of claim 1287, wherein the incorporation of said first nucleotide is accomplished by a terminal transferase or polymerase or a reverse transcriptase.
14109. The method of claim 1398 wherein the polymerase is a Thermococcus sp
151110. The method of claim 14109 wherein the Thermococcus sp is 9°N or a single mutant or double mutant thereof.
161211. The method of claim 151110 wherein the double mutant is -Y409V A485L.
171312. A method for determining the sequence of a target single-stranded polynucleotide, comprising monitoring the sequential incorporation of complementary nucleotides, wherein at least one incorporation is of a nucleotide as defined in any one of claims 62 to 1065 and wherein the identity of the nucleotide incorporated is determined by detecting the label linked to the base, and the blocking group and said label are removed prior to introduction of the next complementary nucleotide.
181413. The method according to claim 171312 wherein the label of the nucleotide and the blocking group are removed in a single chemical treatment step.
191514. The method according to claim 171312, comprising:
(a) providing a plurality of different nucleotides wherein said plurality of different nucleotides are either as defined in any one of claims 62 to 1065 and wherein the detectable label linked to each type of nucleotide can be distinguished upon detection from the detectable label used for other types of nucleotides;
(b) incorporating the nucleotide into the complement of the target single-stranded polynucleotide;
(c) detecting the label of the nucleotide of (b), thereby determining the type of nucleotide incorporated;
(d) removing the label of the nucleotide of (b) and the blocking group; and (e) optionally repeating steps (b)-(d) one or more times;
thereby determining the sequence of a target single-stranded polynucleotide.
201615. The method according to claim 191514, wherein each of the nucleotides are brought into contact with the target sequentially, with removal of non-incorporated nucleotides prior to addition of the next nucleotide, and wherein detection and removal of the label and the blocking group is carried out either after addition of each nucleotide, or after addition of all four nucleotides.
211716. The method according to claim 191514, wherein each of the nucleotides are brought into contact with the target together simultaneously, and non-incorporated nucleotides are removed prior to detection and subsequent to removal of the label and the blocking group.
221817. The method according to claim 191514, comprising a first step and a second step, wherein in the first step, a first composition comprising two of the four nucleotides is brought into contact with the target and non-incorporated nucleotides are removed prior to detection and subsequent to removal of the label, and wherein in the second step, a second composition comprising the two nucleotides not included in the first composition is brought into contact with the target, and non-incorporated nucleotides are removed prior to detection and subsequent tc removal of the label and blocking group, and wherein the first and second steps are optionally repeated one or more times.
231918. The method according to claim 191514, comprising a first step and a second step, wherein in the first step, a composition comprising one of the four nucleotides is brought into contact with the target, and non-incorporated nucleotides are removed prior to detection and subsequent tc removal of the label and blocking group and
wherein in the second step, a second composition comprising the three nucleotides not included in the first composition is brought into contact with the target, and non- incorporated nucleotides are removed prior to detection and subsequent to removal of the label and blocking group and wherein the first step and the second step are optionally repeated one or more times.
242019. The method according to claim 191514, comprising a first step and a second step, wherein in the first step, a first composition comprising three of the four nucleotides is brought into contact with the target, and non-incorporated nucleotides are removed prior to detection and subsequent to removal of the label and blocking group and wherein in the second step, a composition comprising the nucleotide not included in the first composition is brought into contact with the target, and non-incorporated nucleotides are removed prior to detection and subsequent to removal of the label and blocking group and wherein the first step and the second step are optionally repeated one or more times.
252120. A kit, comprising:
(a) a plurality of different nucleotides wherein said plurality of different nucleotides are either as defined in any one of claims 62 to 1065; and
(b) packaging materials therefor.
262221. A kit according to claim 252120, wherein the detectable label in each nucleotide can be distinguished upon detection from the detectable label used for any of the other three types of nucleotide.
272322. The kit of claim 252120 or 262221, further comprising an enzyme and buffers appropriate for the action of the enzyme.
282423. Use of a nucleotide as defined in any one of claims 1 to 1176 in a Sanger or a Sanger- type sequencing method.
292524. An oligonucleotide comprising a modified nucleotide of claims 1-1176.
302625. A nucleotide triphosphate comprising a modified nucleotide of claims 1-1176.
Claim set B - 289 as proposed to be amended unconditinally
[The amendments are in red]
1. A modified nucleotide triphosphate molecule comprising a purine or pyrimidine base and a deoxyribose sugar moiety having a 3’-azidomethyl group.
2. A molecule according to claim 1 wherein said base is linked to a detectable label via a cleavable linker or a non cleavable linker.
3. A molecule according to claim 2 wherein said linker is cleavable.
43. A molecule according to claims 2 or 3 wherein said detectable label is a fluorophore.
5. A molecule according to claims 3 or 4 wherein said linker contains a phosphine cleavable azide.
64. A kit comprising:
(a) four modified nucleotide triphosphate molecules, each comprising a purine or pyrimidine base and a deoxyribose sugar moiety having a 3’-azidomethyl group where each nucleotide has a base that is linked to a detectable label via a cleavable linker and where the detectable label linked to each nucleotide can be distinguished upon detection from the detectable label used for the other three nucleotides; and
(b) packaging materials therefore.
7. A kit according to claim 6 further containing a polymerase.
8. The kit according to claim 7 wherein the polymerase is a Thermococcus sp.
95. A polynucleotide molecule comprising a modified nucleotide comprising a purine or pyrimidine base and a deoxyribose sugar moiety having a 3’-azidomethyl group.
106. A method for determining the sequence of a target single-stranded polynucleotide, comprising monitoring the sequential incorporation of complementary nucleotides, wherein at least one incorporation is of a nucleotide comprising a purine or pyrimidine base and a deoxyribose sugar moiety having a 3’-azidomethyl group where the nucleotide has a base that is linked to a detectable label via a cleavable linker and wherein the identity of the nucleotide is determined by detecting the label linked to the base and the blocking group and label are removed prior to introduction of the next complementary nucleotide.
117. The method of claim 106 wherein the label of the nucleotide and the blocking group are removed in a single chemical treatment step.
128. The method of claims 106 or 117, the method comprising:
(a) providing a plurality of different nucleotides wherein each nucleotide of said plurality of different nucleotides has a 3’-azidomethyl group and a base that is linked to a detectable label via a cleavable linker, wherein the detectable label linked to each type of nucleotide can be distinguished upon detection from the detectable label used for other types of nucleotides;
(b) incorporating the nucleotide into the complement of the target single-stranded polynucleotide;
(c) detecting the label of the nucleotide of (b), thereby determining the type of nucleotide incorporated;
(d) removing the label of the nucleotide of (b) and the blocking group; and
(e) optionally repeating steps (b)-(d) one or more times;
thereby determining the sequence of a target single-stranded polynucleotide.
139. The method of any one of claims 106 to 128 wherein the blocking group is removed using a water soluble phosphine under neutral, aqueous conditions.
14. The method of claim 13 wherein the phosphine is a derivatised trialkyl phosphine.
15. The method of claim 14 wherein the phosphine is derivatised with one or more functionalities selected from the group comprising amino, hydroxyl, carboxyl and sulfonate groups.
Claims set C - 433 claims as proposed to be amended
[The amendments are in red]
1. A kit comprising four modified nucleotide triphosphate molecules, each comprising a purine or pyrimidine base and a deoxyribose sugar moiety wherein the 3' carbon atom of the sugar moiety has attached a group of the structure
-O-Z
wherein Z is of the formula -CH2N3.
2. The kit according to claim 1, comprising the nucleotides A, T, C and G.
3. The kit according to claim 1 or claim 2, further comprising a terminal transferase, polymerase or reverse transcriptase.
4. The kit according to claim 3, comprising a polymerase.
5. The kit according to claim 4, wherein the polymerase is a Thermococcus sp.
6. A method of controlling the incorporation of a nucleotide complementary to a second nucleotide in a target single stranded polynucleotide in a synthesis or sequencing reaction, the method comprising incorporating into a growing complementary polynucleotide a nucleotide comprising a purine or pyrimidine base and a deoxyribose sugar moiety wherein the 3' carbon atom of the sugar moiety has attached a group of the structure-
-O-Z
wherein Z is of the formula -CH2N3,
the incorporation of said nucleotide preventing or blocking introduction of subsequent nucleotide molecules into said growing complementary polynucleotide.
7. The method according to claim 6, wherein four different nucleotides are brought into contact with the target single stranded polynucleotide simultaneously.
8. The method according to claim 6 or claim 7, wherein the 3' -O-CH2N3 is removed from the deoxyribose sugar moiety prior to introduction of the next complementary nucleotide to generate a 3' hydroxyl group.
9. The method of claim 8, wherein the 3'-O-CH2N3 group is removed using a water- soluble phosphine.
10. The method of claim 9, wherein the water-soluble phosphine is a derivatised trialkyl phosphine.
11. The method of claim 10, wherein the derivatised trialkyl phosphine is derivatised with one or more functionalities selected from the group comprising amino, hydroxyl, carboxyl and sultanate groups.
Lists of issues:
The claim numbering in these lists of issues has been adjusted to correspond to the claim sets in this judgment.
Illumina MNP Issues
1) The identity of the Skilled Team;
2) Whether sequencing using reversible chain terminators (RCTs) was common general knowledge at the priority date;
3) Whether claims 1, 12 & 24 of EP  578
578 (claim set A) are obvious in light of:
(claim set A) are obvious in light of:
a) Zavgorodny 1991; or b) Zavgorodny 2000;
4) Whether an insufficiency squeeze operates against Zavgorodny 1991 or 2000;
5) Whether claim 7 of EP  578
578 (claim set A) and claim 6 of EP 433 (claim set C) are invalid for Agrevo-obviousness / insufficiency;
(claim set A) and claim 6 of EP 433 (claim set C) are invalid for Agrevo-obviousness / insufficiency;
6) Whether the proposed amendments to claim 1 of EP  578
578 (claim set A) and claim 9 of EP 289 (claim set B) are bad for added matter;
(claim set A) and claim 9 of EP 289 (claim set B) are bad for added matter;
7) Whether the Modified Nucleotide Patents are entitled to claim priority from GB 0230037;
8) Whether claims 7 and 12 of EP  578
578 (claim set A) and claim 6 of EP 289 (claim set B) are infringed by CoolMPS and whether claim 20 of EP
(claim set A) and claim 6 of EP 289 (claim set B) are infringed by CoolMPS and whether claim 20 of EP  578
578 (claim set A) and claim 4 of EP 289 (claim set B) are infringed by the StandardMPS 2 colour and E variant kits.
(claim set A) and claim 4 of EP 289 (claim set B) are infringed by the StandardMPS 2 colour and E variant kits.
MGI MNP Issues
1. Are each of claims 1, 12 and/or 24 of EP  578
578 (claim set A) obvious over Zavgorodny 1991 and/or Zavgorodny 2000?
(claim set A) obvious over Zavgorodny 1991 and/or Zavgorodny 2000?
2. If the answer to issue 1 is no, is claim 12 of EP  578
578 (claim set A) insufficient on the basis that it covers methods of sequencing using nucleotides, linkers and labels that would not enable the skilled person to perform a sequencing method across the breadth of the claim without undue burden?
(claim set A) insufficient on the basis that it covers methods of sequencing using nucleotides, linkers and labels that would not enable the skilled person to perform a sequencing method across the breadth of the claim without undue burden?
3. Are claim 6 of EP 433 (claim set C) and claim 7 of EP  578
578 (claim set A) obvious for lack of technical contribution, and/or if the answer to issue 1 is no are they insufficient, because they cover a method of controlling the incorporation of a nucleotide having a 3’O-azidomethyl group in a synthesis reaction (not being a sequencing by synthesis reaction)?
(claim set A) obvious for lack of technical contribution, and/or if the answer to issue 1 is no are they insufficient, because they cover a method of controlling the incorporation of a nucleotide having a 3’O-azidomethyl group in a synthesis reaction (not being a sequencing by synthesis reaction)?
4. Is claim 6 of EP 433 (claim set C) obvious for lack of technical contribution, and/or if the answer to issue 1 is no, is it insufficient, because it covers methods of controlling the incorporation of a nucleotide in a sequencing by synthesis reaction in which the nucleotide is neither linked to nor comprises a detectable label?
5. If the answer to issue 1 is no, are each of claims 1, 12 and/or 24 of EP  578
578 (claim set A) insufficient due to lack of enablement/lack of technical contribution?
(claim set A) insufficient due to lack of enablement/lack of technical contribution?
6. Are each of claims 1, 12 and/or 24 of EP  578
578 (claim set A) entitled to claim priority from priority document P2?
(claim set A) entitled to claim priority from priority document P2?
7. If the answer to issue 6 is no, are each of claims 1, 12 and/or 24 (claim set A) obvious over Barnes?
8. Is claim 9 of EP 289 (claim set B) as proposed to be unconditionally amended invalid for added matter?
9. Does the StandardMPS two colour variant and/or DNBSEQ E variant fall within the scope of claim 20 of EP  578
578 (claim set A) and/or claim 4 of EP 289 (claim set B) properly construed?
(claim set A) and/or claim 4 of EP 289 (claim set B) properly construed?
10. Does CoolMPS fall within the scope of claim 12 of EP  578
578 (claim set A) properly construed?
(claim set A) properly construed?
11. If the answer to issue 10 above is no, does CoolMPS infringe claim 12 of EP  578 (claim set A) by equivalence?
578 (claim set A) by equivalence?
12. [Miscellaneous issue in relation to s71 Patents Act 1977]
MGI FP issues (412 and 415)
EP 412
1. Is claim 1 obvious over Buechler?
2. Is claim 1 invalid for added matter?
3. If the answer to issue 2 is yes, does the amendment save claim 1?
4. Does CoolMPS fall within the scope of claim 1 properly construed?
5. If the answer to issue 4 is no, does CoolMPS infringe claim 1 by equivalence?
EP 415
1. Is claim 1 a collocation of two inventions - namely derivatised Dye 2 (as shown at Figure II at [0094] of EP 415) and the azide linker (as synthesised in Example 4 of EP 415) attached to a nucleotide?
2. If so:
a. Is the derivatised Dye 2 obvious over Arnost?
b. Is the azide linker disclosed in or obvious over Milton?
3. Starting from Milton, is there any technical contribution in using derivatised Dye 2 with the azide linker disclosed in Milton?






















